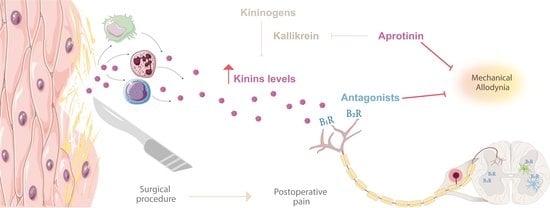
Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals
2.3. Postoperative Pain Model
2.4. Bradykinin-Related Peptides Levels
2.5. Mechanical Allodynia Assessment
2.6. Treatments
2.7. Statistical Analysis
3. Results
3.1. The Surgical Procedure Increases Bradykinin-Related Peptide Levels
3.2. Kinin Receptor Antagonists and a Kallikrein Inhibitor Alleviate the Surgical Procedure-Induced Mechanical Allodynia in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chou, R.; Gordon, D.B.; De Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Commi. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.R.; Vierck, C.J. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J. Pain 2017, 18, 359.e1–359.e38. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from Acute to Chronic Pain after Surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Weiser, T.G.; Hider, P.; Wilson, L.; Gruen, R.L.; Bickler, S.W. Estimated Need for Surgery Worldwide Based on Prevalence of Diseases: A Modelling Strategy for the WHO Global Health Estimate. Lancet Glob. Health 2015, 3, S13–S20. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.S.; Flanagan, S.; Fischberg, D.; Cintron, A.; Siu, A.L. A Novel Interdisciplinary Analgesic Program Reduces Pain and Improves Function in Older Adults after Orthopedic Surgery. J. Am. Geriatr. Soc. 2009, 57, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kehlet, H. Postoperative Pain, Analgesia, and Recovery—Bedfellows That Cannot Be Ignored. Pain 2018, 159, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative Pain—From Mechanisms to Treatment. Pain Rep. 2017, 2, e588. [Google Scholar] [CrossRef]
- Leeb-Lundberg, L.M.F.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: From Molecular Mechanisms to Pathophysiological Consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef] [Green Version]
- Pethö, G.; Reeh, P.W. Sensory and Signaling Mechanisms of Bradykinin, Eicosanoids, Platelet-Activating Factor, and Nitric Oxide in Peripheral Nociceptors. Physiol. Rev. 2012, 92, 1699–1775. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Fialho, M.F.P.; Becker, G.; Brum, E.S.; Favarin, A.; Marquezin, L.P.; Serafini, P.T.; Oliveira, S.M. Kinins and Their B1 and B2 Receptors as Potential Therapeutic Targets for Pain Relief. Life Sci. 2023, 314, 121302. [Google Scholar] [CrossRef]
- Marceau, F.; Regoli, D. Bradykinin Receptor Ligands: Therapeutic Perspectives. Nat. Rev. Drug Discov. 2004, 3, 845–852. [Google Scholar] [CrossRef]
- Cassim, B.; Shaw, O.M.; Mazur, M.; Misso, N.L.; Naran, A.; Langlands, D.R.; Thompson, P.J.; Bhoola, K.D. Kallikreins, Kininogens and Kinin Receptors on Circulating and Synovial Fluid Neutrophils: Role in Kinin Generation in Rheumatoid Arthritis. Rheumatology 2009, 48, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Cialdai, C.; Giuliani, S.; Valenti, C.; Tramontana, M.; Maggi, C.A. Effect of Intra-Articular 4-(S)-Amino-5-(4-{4-[2,4-Dichloro-3-(2,4-Dimethyl-8-Quinolyloxymethyl)Phenylsulfonamido]-Tetrahydro-2h-4-Pyranylcarbonyl} Piperazino)-5-Oxopentyl(Trimethyl)Ammonium Chloride Hydrochloride (MEN16132), a Kinin B2 Receptor Antagoni. J. Pharmacol. Exp. Ther. 2009, 331, 1025–1032. [Google Scholar] [CrossRef]
- Silva, C.R.; Oliveira, S.M.; Hoffmeister, C.; Funck, V.; Guerra, G.P.; Trevisan, G.; Tonello, R.; Rossato, M.F.; Pesquero, J.B.; Bader, M.; et al. The Role of Kinin B 1 Receptor and the Effect of Angiotensin I-Converting Enzyme Inhibition on Acute Gout Attacks in Rodents. Ann. Rheum. Dis. 2016, 75, 260–268. [Google Scholar] [CrossRef]
- Minville, V.; Mouledous, L.; Jaafar, A.; Couture, R.; Brouchet, A.; Frances, B.; Tack, I.; Girolami, J.-P. Tibial Post Fracture Pain Is Reduced in Kinin Receptors Deficient Mice and Blunted by Kinin Receptor Antagonists. J. Transl. Med. 2019, 17, 346. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.C.D.; Vieira, G.; Gonçalves, T.R.; Simões, R.R.; Brusco, I.; Oliveira, S.M.; Calixto, J.B.; Cola, M.; Santos, A.R.S.; Dutra, R.C. Bradykinin Receptors Play a Critical Role in the Chronic Post-Ischaemia Pain Model. Cell. Mol. Neurobiol. 2021, 41, 63–78. [Google Scholar] [CrossRef]
- Swift, J.Q.; Garry, M.G.; Roszkowski, M.T.; Hargreaves, K.M. Effect of Flurbiprofen on Tissue Levels of Immunoreactive Bradykinin and Acute Postoperative Pain. J. Oral Maxillofac. Surg. 1993, 51, 112–116. [Google Scholar] [CrossRef]
- Hamza, M.; Wang, X.M.; Adam, A.; Brahim, J.S.; Rowan, J.S.; Carmona, G.N.; Dionne, R.A. Kinin B1receptors Contributes to Acute Pain Following Minor Surgery in Humans. Mol. Pain 2010, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Leonard, P.A.; Arunkumar, R.; Brennan, T.J. Bradykinin Antagonists Have No Analgesic Effect on Incisional Pain. Anesth. Analg. 2004, 99, 1166–1172. [Google Scholar] [CrossRef]
- Muratani, T.; Doi, Y.; Nishimura, W.; Nishizawa, M.; Minami, T.; Ito, S. Preemptive Analgesia by Zaltoprofen That Inhibits Bradykinin Action and Cyclooxygenase in a Post-Operative Pain Model. Neurosci. Res. 2005, 51, 427–433. [Google Scholar] [CrossRef]
- Füredi, R.; Bölcskei, K.; Szolcsányi, J.; Petho, G. Comparison of the Peripheral Mediator Background of Heat Injury- and Plantar Incision-Induced Drop of the Noxious Heat Threshold in the Rat. Life Sci. 2010, 86, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Silva, C.R.; Trevisan, G.; de Campos Velho Gewehr, C.; Rigo, F.K.; La Rocca Tamiozzo, L.; Rossato, M.F.; Tonello, R.; Dalmolin, G.D.; de Almeida Cabrini, D.; et al. Potentiation of Paclitaxel-Induced Pain Syndrome in Mice by Angiotensin I Converting Enzyme Inhibition and Involvement of Kinins. Mol. Neurobiol. 2017, 54, 7824–7837. [Google Scholar] [CrossRef]
- Brusco, I.; Benatti, A.; Regina, C.; Fischer, S.; Mattar, T.; Scussel, R.; Machado-de-ávila, R.A.; Ferreira, J.; Marchesan, S. Kinins and Their B1 and B2 Receptors Are Involved in Fibromyalgia-like Pain Symptoms in Mice. Biochem. Pharmacol. 2019, 168, 119–132. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing Guidelines on Reporting Research Using Animals (ARRIVE etc.): New Requirements for Publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Drewes, C.C.; Silva, C.R.; Trevisan, G.; Boschen, S.L.; Moreira, C.G.; De Almeida Cabrini, D.; Da Cunha, C.; Ferreira, J. Involvement of Mast Cells in a Mouse Model of Postoperative Pain. Eur. J. Pharmacol. 2011, 672, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Silva, C.R.; Ferreira, J. Critical Role of Protease-Activated Receptor 2 Activation by Mast Cell Tryptase in the Development of Postoperative Pain. Anesthesiology 2013, 118, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Brusco, I.; Camponogara, C.; Carvalho, F.B.; Schetinger, M.R.C.; Oliveira, M.S.; Trevisan, G.; Ferreira, J.; Oliveira, S.M. α-Spinasterol: A COX Inhibitor and a Transient Receptor Potential Vanilloid 1 Antagonist Presents an Antinociceptive Effect in Clinically Relevant Models of Pain in Mice. Br. J. Pharmacol. 2017, 174, 4247–4262. [Google Scholar] [CrossRef] [Green Version]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J. Capturing Novel Non-Opioid Pain Targets. Biol. Psychiatry 2019, 87, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schug, S.A.; Lavand’Homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP Classification of Chronic Pain for ICD-11: Chronic Postsurgical or Posttraumatic Pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.; et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP Classification of Chronic Pain for ICD-11: Chronic Neuropathic Pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A.; et al. Advances in Neuropathic Pain. Arch. Neurol. 2003, 60, 1524. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, M.M.; Gebhart, G.F.; Brennan, T.J. Acute Effect of an Incision on Mechanosensitive Afferents in the Plantar Rat Hindpaw. J. Neurophysiol. 2002, 87, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banik, R.K.; Brennan, T.J. Sensitization of Primary Afferents to Mechanical and Heat Stimuli after Incision in a Novel in Vitro Mouse Glabrous Skin-Nerve Preparation. Pain 2008, 138, 380–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogatzki, E.M.; Gebhart, G.F.; Brennan, T.J. Characterization of Aδ- and C-Fibers Innervating the Plantar Rat Hindpaw One Day after an Incision. J. Neurophysiol. 2002, 87, 721–731. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Dutra, R.C. Kinin Receptors: Key Regulators of Autoimmunity. Autoimmun. Rev. 2017, 16, 192–207. [Google Scholar] [CrossRef]
- Talbot, S.; Chahmi, E.; Dias, J.P.; Couture, R. Key Role for Spinal Dorsal Horn Microglial Kinin B1receptor in Early Diabetic Pain Neuropathy. J. Neuroinflamm. 2010, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Dutra, R.C.; Bento, A.F.; Leite, D.F.P.; Manjavachi, M.N.; Marcon, R.; Bicca, M.A.; Pesquero, J.B.; Calixto, J.B. The Role of Kinin B1 and B2 Receptors in the Persistent Pain Induced by Experimental Autoimmune Encephalomyelitis (EAE) in Mice: Evidence for the Involvement of Astrocytes. Neurobiol. Dis. 2013, 54, 82–93. [Google Scholar] [CrossRef]
- Noda, M.; Kariura, Y.; Amano, T.; Manago, Y.; Nishikawa, K.; Aoki, S.; Wada, K. Expression and Function of Bradykinin Receptors in Microglia. Life Sci. 2003, 72, 1573–1581. [Google Scholar] [CrossRef]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; Cabrini, D.A.; Campos, M.M. Kinin B1 Receptors: Key G-Protein-Coupled Receptors and Their Role in Inflammatory and Painful Processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Raja, S.N. Treatment of Acute Postoperative Pain. Lancet 2011, 377, 2215–2225. [Google Scholar] [CrossRef]
- Sugiura, T.; Tominaga, M.; Mizumura, H.K.K. Bradykinin Lowers the Threshold Temperature for Heat Activation of Vanilloid Receptor 1. J. Neurophysiol. 2002, 88, 544–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, L.; McNaughton, P.A. Proinflammatory Mediators Modulate the Heat-Activated Ion Channel TRPV1 via the Scaffolding Protein AKAP79/150. Neuron 2008, 59, 450–461. [Google Scholar] [CrossRef] [Green Version]




| Preclinical studies | ||||
| Incision plantar model | Behavioural experiments | Treatments | Findings | Reference |
| Longitudinal incision (1 cm) in the skin and fascia of the paw of rats, starting 0.5 cm from the proximal edge of the heel and extending toward the digits. The underlying muscle was dissected and submitted a single ongitudinal incision. | Mechanical hypersensitivity: circular plastic disk (5 mm in diameter) attached to a von electronic Frey filament (400 mN) applied three times in the paw. Five min after this nonpunctate testing, withdrawal responses to punctate mechanical stimulation were determined using von Frey filaments (15, 30, 54, 61, 94, 119, 142, 198, and 522 mN) applied the crescent manner until a withdrawal response or 522 mN (cut-off). Thermal hypersensitivity: withdrawal latencies to heat were assessed by applying a radiant heat source (50-W lamp). | Post-treatment with DALBk or HOE140 (0.1–3.0 mg/kg) intravenous via 2 h or 2 days after incision. Pre-treatment with DALBk or HOE140 (3.0 mg/kg) intravenous via 1 h before incision. One day after the surgical drugs were re-dosed. | None effect on mechanical or heat hypersensitivity evaluated from 0.5 to 2.5 h after treatments (post-treatment) or 2 h after incision or 1 h after re-dose (pre-treatment). | Leonard et al. (2004) [19]. |
| Longitudinal incision (5 mm) in the skin and fascia of the paw mice and muscle was elevated and incised longitudinally. | Mechanical hypersensitivity: von Frey filaments were applied to the paw at an increasing force until the mouse withdrew its hind limb. | [des-Arg10]-HOE140 (0.2 mg/kg) and HOE140 (0.002–0.2 mg/kg) administered subcutaneously 30 min before incision. HOE140 (0.002—0.2 mg/kg) administered 5 min after the surgical procedure. | HOE140, but not [des-Arg10]-HOE140, reduced the mechanical hypersensitivity dose dependent from 2 h to 3 days after the operation (pretreatment). HOE140 (0.2 mg/kg), reduced mechanical hypersensitivity at 2 h after operation (post-treatment). None change kinin B1 and B2 receptors mRNA expression in the plantar tissue or spinal cord 24 h after the operation, or after pre-treatment with HOE140. | Muratani et al., 2005 [20]. |
| The incision started 1 cm from the proximal edge of the heel of rats and extended longitudinally 1 cm toward the toes, intersecting the skin, fascia and plantar muscle. | Thermal hypersensitivity: noxious heat threshold temperature was determined in an increasing-temperature water bath initiating at 30 °C with a heating rate of 24 °C/min and cut-off of 53 °C. | [des-Arg10]-HOE140 and HOE140 (10 µM) intraplantar via 18 h after operation. | [des-Arg10]-HOE140 and HOE140 alleviated the heat hypersensitivity from 10 to 40 min after treatment. | Füredi et al., 2010 [21]. |
| Longitudinal incision (5 mm) in the skin and fascia of the paw of mice and elevation of the underlying muscle. The incision started 2 mm from the proximal edge of the heel and extended toward the toes. | Mechanical hypersensitivity: manual von Frey filaments of increasing stiffness (0.02, 0.07, 0.16, 0.4, 1.4, 4.0 and 10.0 g). Six measurements were performed alternating the filaments conforming Oliveira et al., 2011 [27]; 2013 [28]; Brusco et al., 2017 [29]. | DALBk (10–100 nmol/kg), Icatibant (HOE140; 300–1000 nmol/kg), SSR240612 (100–1000 nmol/kg) or FR173657 (30–300 nmol/kg), subcutaneous via 0.5 h before surgery DALBk (0.1–1 nmol/paw), Icatibant (HOE140; 1, 3, and 10 nmol/paw) or Aprotinin (100 µg/paw) intraplantar via 10 min before the surgery. | DALBk, Icatibant (HOE140), SSR240612 and FR173657 (systemic) or DALBk, Icatibant (HOE140) and Aprotinin (local) reduced the mechanical allodynia up to a maximum of 2 h after surgical. Bradykinin-related peptide levels increased in the serum and paw tissue. | Data of present study |
| Clinical studies | ||||
| Findings | Reference | Findings | Reference | |
| Increased kinin levels in the microdialysate samples obtained from after oral surgery in humans. | Swift et al., 1993 [17]. | Increased kinin B1 and B2 receptor mRNA expression and kinin levels in mucosal biopsies three hours after oral surgery in humans. | Hamza et al., 2010 [18]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusco, I.; Silva, C.R.; Ferreira, J.; Oliveira, S.M. Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications. Brain Sci. 2023, 13, 941. https://doi.org/10.3390/brainsci13060941
Brusco I, Silva CR, Ferreira J, Oliveira SM. Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications. Brain Sciences. 2023; 13(6):941. https://doi.org/10.3390/brainsci13060941
Chicago/Turabian StyleBrusco, Indiara, Cássia Regina Silva, Juliano Ferreira, and Sara Marchesan Oliveira. 2023. "Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications" Brain Sciences 13, no. 6: 941. https://doi.org/10.3390/brainsci13060941
APA StyleBrusco, I., Silva, C. R., Ferreira, J., & Oliveira, S. M. (2023). Kinins’ Contribution to Postoperative Pain in an Experimental Animal Model and Its Implications. Brain Sciences, 13(6), 941. https://doi.org/10.3390/brainsci13060941