History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair
Abstract
:1. SCI Background and Need for Therapies
2. Discovery of GDNF Family Ligands and Receptors
3. Localization of GDNF and its Receptors
4. GDNF Promotes Cell Survival and Growth
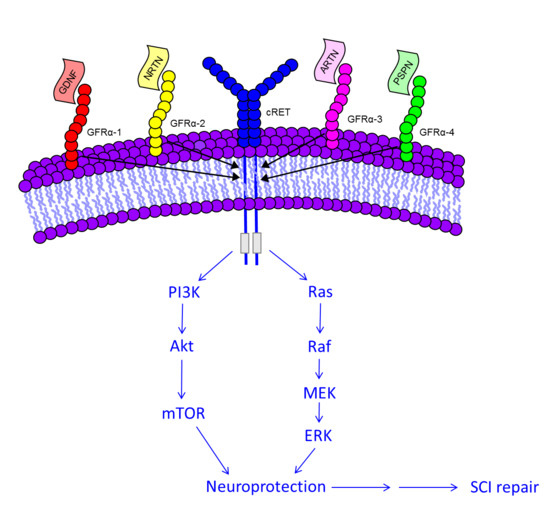
5. Molecular Signaling of GDNF for Promotion of Cell Survival
6. Studies Employing GDNF for Repair of Experimental SCI
7. Studies Using GDNF in Combinational Therapies for Experimental SCI Repair
Author Contributions
Funding
Conflicts of Interest
References
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, L.J.; Wrathall, J.R. The blood-spinal cord barrier after injury: Pattern of vascular events proximal and distal to a transection in the rat. Brain Res. 1987, 424, 177–188. [Google Scholar] [CrossRef]
- Popovich, P.G.; Horner, P.J.; Mullin, B.B.; Stokes, B.T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996, 142, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Schnell, L.; Fearn, S.; Klassen, H.; Schwab, M.E.; Perry, V.H. Acute inflammatory responses to mechanical lesions in the CNS: Differences between brain and spinal cord. Eur. J. Neurosci. 1999, 11, 3648–3658. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansorena, E.; De Berdt, P.; Ucakar, B.; Simón-Yarza, T.; Jacobs, D.; Schakman, O.; Jankovski, A.; Deumens, R.; Blanco-Prieto, M.J.; Préat, V.; et al. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int. J. Pharm. 2013, 455, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.-X.; Hu, J.; Liu, N.; Wang, X.; Smith, G.M.; Wen, X.; Xu, X.-M. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp. Neurol. 2011, 229, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horger, B.A.; Nishimura, M.C.; Armanini, M.P.; Wang, L.C.; Poulsen, K.T.; Rosenblad, C.; Kirik, D.; Moffat, B.; Simmons, L.; Johnson, E.; et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J. Neurosci. 1998, 18, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Raynoschek, C.; Belluardo, N.; Ibáñez, C.F. Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol. Cell. Neurosci. 1998, 11, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim. Biophys. Acta 2013, 1834, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Widenfalk, J.; Wu, W.; Hao, J.; Person, J.K.E.; Wiesenfeldt-Hallin, Z.; Risling, M. Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 2009, 43, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.E.; Gibson, M.E.; Arnold, H.M.; Pepinsky, B.; Frank, E. Artemin promotes functional long-distance axonal regeneration to the brainstem after dorsal root crush. Proc. Natl. Acad. Sci. USA 2015, 112, 6170–6175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buj-Bello, A.; Adu, J.; Piñón, L.G.; Horton, A.; Thompson, J.; Rosenthal, A.; Chinchetru, M.; Buchman, V.L.; Davies, A.M. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature 1997, 387, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.P.; Milbrandt, J.; Johnson, E.M. Neurturin and persephin promote the survival of embryonic basal forebrain cholinergic neurons in vitro. Exp. Neurol. 2003, 184, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, J.; de Sauvage, F.J.; Fahrner, T.J.; Baloh, R.H.; Leitner, M.L.; Tansey, M.G.; Lampe, P.A.; Heuckeroth, R.O.; Kotzbauer, P.T.; Simburger, K.S.; et al. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 1998, 20, 245–253. [Google Scholar] [CrossRef]
- Tomac, A.C.; Agulnick, A.D.; Haughey, N.; Chang, C.-F.; Zhang, Y.; Bäckman, C.; Morales, M.; Mattson, M.P.; Wang, Y.; Westphal, H.; et al. Effects of cerebral ischemia in mice deficient in Persephin. Proc. Natl. Acad. Sci. USA 2002, 99, 9521–9526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engele, J.; Schubert, D.; Bohn, M.C. Conditioned media derived from glial cell lines promote survival and differentiation of dopaminergic neurons in vitro: Role of mesencephalic glia. J. Neurosci. Res. 1991, 30, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Wen, D.; Yu, Y.; Holst, P.L.; Luo, Y.; Fang, M.; Tamir, R.; Antonio, L.; Hu, Z.; Cupples, R.; et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 1996, 85, 1113–1124. [Google Scholar] [CrossRef]
- Treanor, J.J.; Goodman, L.; de Sauvage, F.; Stone, D.M.; Poulsen, K.T.; Beck, C.D.; Gray, C.; Armanini, M.P.; Pollock, R.A.; Hefti, F.; et al. Characterization of a multicomponent receptor for GDNF. Nature 1996, 382, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Belluardo, N.; Funakoshi, H.; Ibáñez, C.F. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 1997, 17, 3554–3567. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Arenas, E.; Fainzilber, M.; Nilsson, A.S.; Sieber, B.A.; Grigoriou, M.; Kilkenny, C.; Salazar-Grueso, E.; Pachnis, V.; Arumäe, U. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 1996, 381, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Nicole, O.; Ali, C.; Docagne, F.; Plawinski, L.; MacKenzie, E.T.; Vivien, D.; Buisson, A. Neuroprotection mediated by glial cell line-derived neurotrophic factor: Involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J. Neurosci. 2001, 21, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.W.; Fisher, C.E.; Perona-Wright, G.; Davies, J.A. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J. Cell Sci. 2002, 115, 4495–4503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, K.; Matsuyama, Y.; Kamiya, M.; Kawakami, H.; Iwata, H.; Adachi, K.; Kiuchi, K. Up-regulation of glial cell line-derived neurotrophic factor (GDNF) following traumatic spinal cord injury. Neuroreport 2000, 11, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, M.C. Motoneurons crave glial cell line-derived neurotrophic factor. Exp. Neurol. 2004, 190, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Trok, K.; Hoffer, B.; Olson, L. Glial cell line-derived neurotrophic factor enhances survival and growth of prenatal and postnatal spinal cord transplants. Neuroscience 1996, 71, 231–241. [Google Scholar] [CrossRef]
- Klöcker, N.; Bräunling, F.; Isenmann, S.; Bähr, M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport 1997, 8, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.J.; Saldanha, G.J.; Kennedy, A.J.; Facer, P.; Birch, R.; Carlstedt, T.; Anand, P. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. Neuroreport 1998, 9, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Höke, A.; Gordon, T.; Zochodne, D.W.; Sulaiman, O.A.R. A Decline in Glial Cell-Line-Derived Neurotrophic Factor Expression Is Associated with Impaired Regeneration after Long-Term Schwann Cell Denervation. Exp. Neurol. 2002, 173, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hase, A.; Saito, F.; Yamada, H.; Arai, K.; Shimizu, T.; Matsumura, K. Characterization of glial cell line-derived neurotrophic factor family receptor alpha-1 in peripheral nerve Schwann cells. J. Neurochem. 2005, 95, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ Transplant. 2013, 18, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.; Pollock, R.A.; Philippe, J.M.; Pennica, D.; Henderson, C.E.; deLapeyrière, O. Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1). J. Neurosci. 1998, 18, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, R.W.; Houenou, L.J.; Johnson, J.E.; Lin, L.F.; Li, L.; Lo, A.C.; Newsome, A.L.; Prevette, D.M.; Wang, S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 1995, 373, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Rind, H.B.; von Bartheld, C.S. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. Neuroreport 2002, 13, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Z.; Qiu, G.; Song, Y. Combination of chondroitinase ABC, glial cell line-derived neurotrophic factor and Nogo A antibody delayed-release microspheres promotes the functional recovery of spinal cord injury. J. Craniofac. Surg. 2013, 24, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Valverde, J.; Alexi, T.; Poulsen, K.; Moffat, B.; Vandlen, R.A.; Rosenthal, A.; Hefti, F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 1995, 373, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Tomac, A.; Lindqvist, E.; Lin, L.F.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.H.; Liu, R.Y.; Zhang, Y.; Sun, H.R.; Qin, L.Y.; Lau, L.T.; Wu, B.Y.; Hui, H.K.; Heung, M.Y.; Han, J.S. Glial cell line-derived neurotrophic factor protects astrocytes from staurosporine- and ischemia- induced apoptosis. J. Neurosci. Res. 2007, 85, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-F.; Wang, W.-T.; Ghanta, V.K.; Lin, C.-H.; Chiang, Y.-Y.; Hsueh, C.-M. Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res. 2008, 1239, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Kilic, E.; Kügler, S.; Isenmann, S.; Bähr, M. Adenovirus-mediated glial cell line-derived neurotrophic factor (GDNF) expression protects against subsequent cortical cold injury in rats. Neurobiol. Dis. 2011, 8, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Nakajima, K.; Nakamura, Y.; Imai, Y.; Kohsaka, S. Rat primary cultured microglia express glial cell line-derived neurotrophic factor receptors. Neurosci. Lett. 1999, 275, 203–206. [Google Scholar] [CrossRef]
- Iravani, M.M.; Sadeghian, M.; Leung, C.C.M.; Jenner, P.; Rose, S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1β tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci. Lett. 2012, 510, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.M.; Dolcet, X.; Encinas, M.; Egea, J.; Bayascas, J.R.; Comella, J.X. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J. Neurosci. 1999, 19, 9160–9169. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Shaymardanova, G.F.; Garanina, E.E.; Salafutdinov, I.I.; Rizvanov, A.A.; Islamov, R.R.; Chelyshev, Y.A. Adenoviral vector carrying glial cell-derived neurotrophic factor for direct gene therapy in comparison with human umbilical cord blood cell-mediated therapy of spinal cord injury in rat. Spinal Cord 2016, 54, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, J.-P.; Tzeng, S.-F. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J. Neurosci. Res. 2002, 69, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, W.; Lin, L.F.; Lei, M.; Oppenheim, R.W.; Houenou, L.J. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 9771–9775. [Google Scholar] [CrossRef] [PubMed]
- Watabe, K.; Ohashi, T.; Sakamoto, T.; Kawazoe, Y.; Takeshima, T.; Oyanagi, K.; Inoue, K.; Eto, Y.; Kim, S.U. Rescue of lesioned adult rat spinal motoneurons by adenoviral gene transfer of glial cell line-derived neurotrophic factor. J. Neurosci. Res. 2000, 60, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Ramer, M.S.; Bradbury, E.J.; Michael, G.J.; Lever, I.J.; McMahon, S.B. Glial cell line-derived neurotrophic factor increases calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur. J. Neurosci. 2003, 18, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Allchorne, A.J.; Griffin, R.S.; Woolf, C.J.; Costigan, M. GDNF selectively promotes regeneration of injury-primed sensory neurons in the lesioned spinal cord. Mol. Cell. Neurosci. 2007, 36, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, C.-H.; Chen, S.-H.; Chio, C.-C.; Chang, C.-K.; Lin, M.-T. Exogenous administration of glial cell line-derived neurotrophic factor improves recovery after spinal cord injury. Resuscitation 2008, 77, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, C.; Li, H.; Yan, P.; Lu, X.; Wirthlin, L.; Xu, X.M. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp. Neurol. 2003, 183, 379–393. [Google Scholar] [CrossRef]
- Blesch, A.; Tuszynski, M.H. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J. Comp. Neurol. 2003, 467, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Dolbeare, D.; Houle, J.D. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF). J. Neurotrauma 2003, 20, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Z.; Smith, G.M.; Wen, X.; Pressman, Y.; Wood, P.M.; Xu, X.-M. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 2009, 57, 1178–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Alam, S.; Oppenheim, R.W.; Prevette, D.M.; Evenson, A.; Parsadanian, A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp. Neurol. 2004, 190, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-X.; Deng, P.; Ruan, Y.; Xu, Z.C.; Liu, N.-K.; Wen, X.; Smith, G.M.; Xu, X.-M. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J. Neurosci. 2013, 33, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2018, 12, e398–e407. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaie, M.; Mansour, R.N.; Nazari, B.; Hassannia, H.; Hosseini, F.; Mahboudi, H.; Enderami, S.E. Improved stem cell therapy of spinal cord injury using GDNF-overexpressed bone marrow stem cells in a rat model. Biol. J. Int. Assoc. Biol. Stand. 2017, 50, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Lou, G.; Mo, Y.; Pan, Y.; Zhang, Z.; Guo, R.; Li, Z. A combination of GDNF and hUCMSC transplantation loaded on SF/AGs composite scaffolds for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, H.; Zhang, M.; Chen, B.; Yang, H. Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 1800–1811. [Google Scholar] [CrossRef] [Green Version]

| Ligand | Receptor | Co-Receptor |
|---|---|---|
| GDNF | GFRα 1 | cRET |
| NRTN | GFRα 2 | cRET |
| ARTN | GFRα 3 | cRET |
| PSPN | GFRα 4 | cRET |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, M.J.; Xu, X.-M. History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair. Brain Sci. 2018, 8, 109. https://doi.org/10.3390/brainsci8060109
Walker MJ, Xu X-M. History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair. Brain Sciences. 2018; 8(6):109. https://doi.org/10.3390/brainsci8060109
Chicago/Turabian StyleWalker, Melissa J., and Xiao-Ming Xu. 2018. "History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair" Brain Sciences 8, no. 6: 109. https://doi.org/10.3390/brainsci8060109
APA StyleWalker, M. J., & Xu, X.-M. (2018). History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair. Brain Sciences, 8(6), 109. https://doi.org/10.3390/brainsci8060109