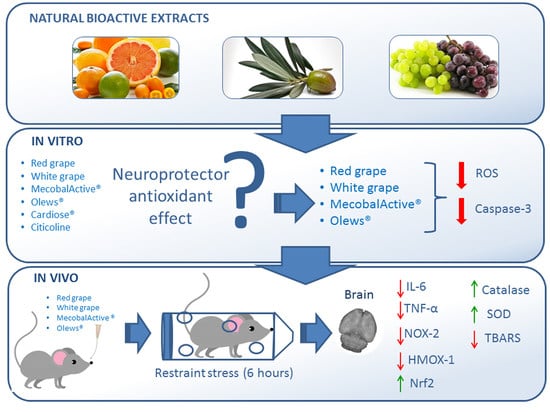
Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Primary Hippocampal Neuron Isolation and Culture
2.3. Natural Extracts
2.4. Preparation of Aluminum Maltolate
2.5. Cell Proliferation Assay
2.6. Measurement of Intracellular ROS Levels
2.7. Caspase-3 Activation Assay
2.8. Measurement of Nitrite and Nitrate Concentrations
2.9. Restrain Stress and In Vivo Treatments
2.10. Quantitative Real-Time PCR
2.11. Thiobarbituric Acid Reactive Substances (TBARS), Superoxide Dismutase (SOD), and Catalase Activity
2.12. Statistical Analysis
3. Results
3.1. Olews® and Red and White Grape Extracts Have Neuroprotective Effects on the SH-SY 5Y Cell Line
3.2. Olews®, MecobalActive®, and Red and White Grape Extracts Have Neuroprotective Effects on Neuroblasts In Vitro
3.3. MecobalActive®, Olews®, and Red and White Grape Extracts Treatment Reduces ROS Levels and Caspase-3 Activity
3.4. Immobilization for Six h Causes Oxidative Stress in Mouse Brains
3.5. Oral Administration of Natural Extracts Provides Protection against Oxidative Stress
3.6. Preventive Treatment with Natural Extracts Increases Antioxidant Enzyme Activity in the Brain
3.7. Treatment with Natural Extracts Prevents the Formation of Lipid Peroxidation Products in the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [Green Version]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Troster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.I.; Lee, H.W.; Eom, T.M.; Lim, S.A.; Ha, H.Y.; Seol, I.C.; Kim, Y.S.; Oh, D.S.; Yoo, H.R. A traditional Korean multiple herbal formulae (Yuk-Mi-Jihwang-Tang) attenuates acute restraint stress-induced brain tissue oxidation. Drug Chem. Toxicol. 2017, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Maccarrone, M. Chronic inflammatory disorders and their redox control: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 2605–2641. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.P.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kwon, D.Y.; Cho, H.J.; Lee, M.S. Protective effects of Korean herbal remedy against oxidative stress in cardiomyocytes. Phytother. Res. 2006, 20, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Yen, C.C.; Huang, Y.L.; Pan, C.H.; Lu, F.J.; Chao, M.R. Oxidatively damaged DNA induced by humic acid and arsenic in maternal and neonatal mice. Chemosphere 2010, 79, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Nie, Y.; Zhang, X.; Tan, B.; Cao, H.; Chen, W.; Gao, W.; Chen, J.; Liang, Z.; Lai, H.; et al. Effects of grape seed proanthocyanidin on Alzheimer’s disease in vitro and in vivo. Exp. Ther. Med. 2016, 12, 1681–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Duraes, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Perez, C.; Garcia-Villanova, B.; Guerra-Hernandez, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Sim, D.A.; Miller, J.H. Effect of taxoid and nontaxoid site microtubule-stabilizing agents on axonal transport of mitochondria in untransfected and ECFP-htau40-transfected rat cortical neurons in culture. J. Neurosci. Res. 2014, 92, 1155–1166. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Zaldivar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Avila, E.; Chavarria, A. A Potential Alternative against Neurodegenerative Diseases: Phytodrugs. Oxid. Med. Cell. Longev. 2016, 2016, 8378613. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2020, 120, 159–235. [Google Scholar] [PubMed]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupre, H.; Castonguay, A.M.; Gora, C.; Amri, M.; Lévesque, M. Neuroprotective benefits of grape seed and skin extract in a mouse model of Parkinson’s disease. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.W. Isolation and culture of hippocampal neurons from prenatal mice. J. Vis. Exp. 2012, e3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavente-Garcia, O.; Castillo, J.; Alcaraz, M.; Vicente, V.; Del Rio, J.A. Beneficial action of Citrus flavonoids on multiple cancer-related biological pathways. Curr. Cancer Drug Targets 2007, 7, 795–809. [Google Scholar]
- Bertholf, R.L.; Herman, M.M.; Savory, J.; Carpenter, R.M.; Sturgill, B.C.; Katsetos, C.D.; VandenBerg, S.R.; Wills, M.R. A long-term intravenous model of aluminum maltol toxicity in rabbits: Tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol. Appl. Pharmacol. 1989, 98, 58–74. [Google Scholar] [CrossRef]
- Bomfim, L.M.; de Araujo, F.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Correa, R.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. Ruthenium(II) complexes with 6-methyl-2-thiouracil selectively reduce cell proliferation, cause DNA double-strand break and trigger caspase-mediated apoptosis through JNK/p38 pathways in human acute promyelocytic leukemia cells. Sci. Rep. 2019, 9, 11483. [Google Scholar] [CrossRef]
- Perez Nievas, B.G.; Hammerschmidt, T.; Kummer, M.P.; Terwel, D.; Leza, J.C.; Heneka, M.T. Restraint stress increases neuroinflammation independently of amyloid beta levels in amyloid precursor protein/PS1 transgenic mice. J. Neurochem. 2011, 116, 43–52. [Google Scholar] [CrossRef]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress 2017, 6, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zheng, Y.; Wu, T.; Wu, C.; Cheng, X. Oral administration of grape seed polyphenol extract restores memory deficits in chronic cerebral hypoperfusion rats. Behav. Pharmacol. 2017, 28, 207–213. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, H.; Cao, K.; Sun, D.; Yang, Y.; Liu, C.; Cui, J.; Cheng, Y.; Li, B.; Cai, J.; et al. Radioprotective Effect of Grape Seed Proanthocyanidins In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2016, 2016, 5706751. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Qian, M.; Shi, K.; Chen, G.; Gu, Y.; Du, W.; Zhu, G. Restorative effect and mechanism of mecobalamin on sciatic nerve crush injury in mice. Neural Regen. Res. 2014, 9, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Kimura, Y. Effects of olive leaf extract and its main component oleuroepin on acute ultraviolet B irradiation-induced skin changes in C57BL/6J mice. Phytother. Res. 2010, 24, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Iniguez, J.; Martínez, A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad Rather, M.; Justin Thenmozhi, A.; Manivasagam, T.; Nataraj, J.; Essa, M.M.; Chidambaram, S.B. Asiatic acid nullified aluminium toxicity in in vitro model of Alzheimer’s disease. Front. Biosci. 2018, 10, 287–299. [Google Scholar]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Levonen, A.L.; Inkala, M.; Heikura, T.; Jauhiainen, S.; Jyrkkänen, H.K.; Kansanen, E.; Määttä, K.; Romppanen, E.; Turunen, P.; Rutanen, J.; et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 741–747. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, V.D.; Deshmukh, S.V. Stress-adaptation failure hypothesis of Alzheimer’s disease. Med. Hypotheses 1990, 32, 293–295. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Hou, M.F.; Yang, Z.W.; Tang, J.Y.; Li, K.T.; Huang, H.W.; Huang, Y.H.; Lee, S.Y.; Fu, T.F.; Hsieh, C.Y.; et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, D.M.; Rocha, C.V.J.; da Silveira, E.F.; Marinho, M.A.G.; Rodrigues, M.R.; Silva, N.O.; Ferreira, A.S.; Fernandes de Moura, N.; Sagrera Darelli, G.J.; Braganhol, E.; et al. In Vitro Anti/Pro-oxidant Activities of R. ferruginea Extract and Its Effect on Glioma Cell Viability: Correlation with Phenolic Compound Content and Effects on Membrane Dynamics. J. Membr. Biol. 2018, 251, 247–261. [Google Scholar] [CrossRef]
- Nemeth, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Jin, H.; Tan, X.; Liu, X.; Ding, Y. The study of effect of tea polyphenols on microsatellite instability colorectal cancer and its molecular mechanism. Int. J. Colorectal Dis. 2010, 25, 1407–1415. [Google Scholar] [CrossRef]
- Corona, G.; Tzounis, X.; Assunta Dessi, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef]
- Ciafardini, G.; Marsilio, V.; Lanza, B.; Pozzi, N. Hydrolysis of Oleuropein by Lactobacillus plantarum Strains Associated with Olive Fermentation. Appl. Environ. Microbiol. 1994, 60, 4142–4147. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.; Paiva-Martins, F.; Corona, G.; Debnam, E.S.; Jose Oruna-Concha, M.; Vauzour, D.; Gordon, M.H.; Spencer, J.P.E. Absorption and metabolism of olive oil secoiridoids in the small intestine. Br. J. Nutr. 2011, 105, 1607–1618. [Google Scholar] [CrossRef] [Green Version]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.; Macia, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.D.; Yin, J.H.; Hwang, C.S.; Tang, C.M.; Yang, D.I. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: Implication in Alzheimer’s disease and cerebral ischemia. Free Radic. Res. 2012, 46, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.K.; Gray, J.J.; Winter, A.N.; Linseman, D.A. Immunocal(R) and preservation of glutathione as a novel neuroprotective strategy for degenerative disorders of the nervous system. Recent Pat. CNS Drug Discov. 2012, 7, 230–235. [Google Scholar]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Stocchetti, N.; Pagan, F.; Calappi, E.; Canavesi, K.; Beretta, L.; Citerio, G.; Cormio, M.; Colombo, A. Inaccurate early assessment of neurological severity in head injury. J. Neurotrauma 2004, 21, 1131–1140. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Haiar, J.M.; Watt, M.J.; Newton, S.S. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress 2017, 20, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sulakhiya, K.; Patel, V.K.; Saxena, R.; Dashore, J.; Srivastava, A.K.; Rathore, M. Effect of Beta vulgaris Linn. Leaves Extract on Anxiety- and Depressive-like Behavior and Oxidative Stress in Mice after Acute Restraint Stress. Pharmacognosy Res. 2016, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Garg, R.; Gaur, V.; Kumar, P. Possible role of NO modulators in protective effect of trazodone and citalopram (antidepressants) in acute immobilization stress in mice. Indian J. Exp. Biol. 2010, 48, 1131–1135. [Google Scholar]
- Duval, C.; Cantero, A.V.; Auge, N.; Mabile, L.; Thiers, J.C.; Negre-Salvayre, A.; Salvayre, R. Proliferation and wound healing of vascular cells trigger the generation of extracellular reactive oxygen species and LDL oxidation. Free Radic. Biol. Med. 2003, 35, 1589–1598. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Agúndez, J.A.; García-Martín, E.; Martínez, C.; Benito-León, J.; Millán-Pascual, J.; Díaz-Sánchez, M.; Calleja, P.; Pisa, D.; Turpín-Fenoll, L.; Alonso-Navarro, H.; et al. Heme Oxygenase-1 and 2 Common Genetic Variants and Risk for Multiple Sclerosis. Sci. Rep. 2016, 6, 20830. [Google Scholar] [CrossRef] [Green Version]
- Emerson, M.R.; LeVine, S.M. Heme oxygenase-1 and NADPH cytochrome P450 reductase expression in experimental allergic encephalomyelitis: An expanded view of the stress response. J. Neurochem. 2000, 75, 2555–2562. [Google Scholar] [CrossRef]
- Van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.D.; van der Valk, P.; de Vries, H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, M.; Castilla-Ortega, E.; Pedraza, C.; Blanco, E.; Hurtado-Guerrero, I.; Barbancho, M.A.; Chun, J.; Rodríguez-de-Fonseca, F.; Estivill-Torrús, G.; Santín Núñez, L.J. Chronic immobilization in the malpar1 knockout mice increases oxidative stress in the hippocampus. Int. J. Neurosci. 2012, 122, 583–589. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Kowalczuk, K.; Stryjecka-Zimmer, M. The influence of oxidative stress on the level of malondialdehyde (MDA) in different areas of the rabbit brain. Ann. Univ. Mariae Curie Sklodowska Med. 2002, 57, 160–164. [Google Scholar]







| Natural Extract | Dose | References |
|---|---|---|
| Red grape | 100 mg/kg | [29,30] |
| 300 mg/kg | ||
| White grape | 100 mg/kg | [29,30] |
| 300 mg/kg | ||
| MecobalActive® | 65 µg/kg | [31] |
| 135 µg/kg | ||
| Olews® | 300 mg/kg | [32,33] |
| 600 mg/kg |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| NOX-2 | GCTGGGATCACAGGAATTGT | CTTCCAAACTCTCCGCAGTC |
| HMOX-1 | TGCTCGAATGAACACTCTGG | TAGCAGGCCTCTGACGAAGT |
| IL-6 | ATGGATGCTACCAAACTGGAT | TGAAGGACTCTGGCTTTGTCT |
| TNF-alpha | CCACCACGCTCTTCTGTCTA | CACTTGGTGGTTTGCTACGA |
| Nrf-2 | AGCGAGCAGGCTATCTCCTA | TCTTGCCTCCAAAGGATGTC |
| GAPDH | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobadilla, M.; García-Sanmartín, J.; Martínez, A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants 2021, 10, 46. https://doi.org/10.3390/antiox10010046
Bobadilla M, García-Sanmartín J, Martínez A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants. 2021; 10(1):46. https://doi.org/10.3390/antiox10010046
Chicago/Turabian StyleBobadilla, Miriam, Josune García-Sanmartín, and Alfredo Martínez. 2021. "Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases" Antioxidants 10, no. 1: 46. https://doi.org/10.3390/antiox10010046
APA StyleBobadilla, M., García-Sanmartín, J., & Martínez, A. (2021). Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants, 10(1), 46. https://doi.org/10.3390/antiox10010046