Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene
Abstract
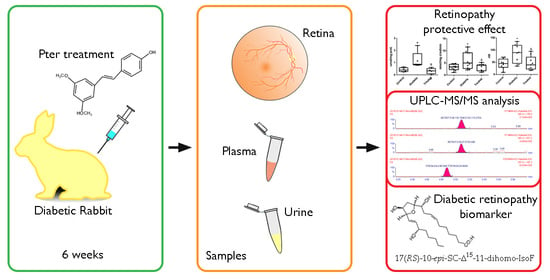
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Standards and Reagents
2.3. Retina Sample Analysis
2.4. Plasma Sample Analysis
2.5. Urine Sample Analysis
2.6. UPLC-MS/MS Analysis
2.7. Statistical Analyses
3. Results
3.1. Pterostilbene Reduces Harmful Retinal Lipid Oxidation Induced by Chronic Hyperglycemia In Vivo
3.2. Determination of Lipid Oxidation Analytes in Plasma and Urine in an Experimental Diabetes Type 1 Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nigel Unwin, D.G.; Mbanya, J.C.; Ramachandran, A.; Roglic, G.; Shaw, J.; Soltèsz, G.; Whiting, D.; Zgibor, J.; Zhang, P.; Zimmet, P.; et al. IDF Diabets Atlas Fourth Edition. Available online: www.eatlas.idf.org (accessed on 10 December 2020).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell Longev. 2019, 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and vascular complications of Diabetes: An update. Phytomedicine 2019, 56, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Millán, I.; Desco, M.D.C.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene prevents early diabetic retinopathy alterations in a rabbit experimental model. Nutrients 2020, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Dodda, D.; Rama Rao, A.; Veeresham, C. In vitro and in vivo evaluation of pterostilbene for the management of diabetic complications. J. Ayurveda Integr. Med. 2020, 11, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Thangasamy, G.; Pari, L. Protective role of pterostilbene on plasma andtissue glycoprotein components in high-fat diet-fed and streptozotocin-inducedtype 2 diabetic mice. Asian J. Pharm. Clin. Res. 2019, 12, 4. [Google Scholar] [CrossRef]
- Berdeaux, O.; Acar, N. Very-long-chain polyunsaturated fatty acids in the retina: Analysis and clinical relevance in physiological and pathological conditions. Oilseeds Fat Crops Lipids 2011, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Agbaga, M.P.; Mandal, M.N.; Anderson, R.E. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J. Lipid Res. 2010, 51, 1624–1642. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A.; Krohne, T.U.; Sapieha, P.; Chen, J.; Hellstrom, A.; Chew, E.; Holz, F.G.; Smith, L.E. Lipid metabolites in the pathogenesis and treatment of neovascular eye disease. Br. J. Ophthalmol. 2011, 95, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Eye Res. 2010, 90, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, J.A.; Miranda, F.J.; Lloréns, S.; Centeno, J.M.; Marrachelli, V.G.; Alborch, E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur. J. Pharmacol. 2004, 486, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Torre, A.; Lee, Y.Y.; Mazzoni, A.; Guy, A.; Bultel-Poncé, V.; Durand, T.; Oger, C.; Lee, J.C.; Galano, J.M. Total syntheses and in vivo quantitation of novel neurofuran and dihomo-isofuran derived from docosahexaenoic acid and adrenic acid. Chemistry 2015, 21, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Cháfer-Pericás, C.; Rahkonen, L.; Sánchez-Illana, A.; Kuligowski, J.; Torres-Cuevas, I.; Cernada, M.; Cubells, E.; Nuñez-Ramiro, A.; Andersson, S.; Vento, M.; et al. Ultra high performance liquid chromatography coupled to tandem mass spectrometry determination of lipid peroxidation biomarkers in newborn serum samples. Anal. Chim. Acta 2015, 886, 214–220. [Google Scholar] [CrossRef]
- Bioanalytical Method Validation. Guidance for Industry; US Department of Health and Human Services; Food and Drug Administration (FDA); Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM); Silver Spring: Montgomery County, MD, USA, 2018; p. 44. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 10 December 2020).
- García-Blanco, A.; Peña-Bautista, C.; Oger, C.; Vigor, C.; Galano, J.M.; Durand, T.; Martín-Ibáñez, N.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta 2018, 184, 193–201. [Google Scholar] [CrossRef]
- Kuligowski, J.; Escobar, J.; Quintás, G.; Lliso, I.; Torres-Cuevas, I.; Nuñez, A.; Cubells, E.; Rook, D.; van Goudoever, J.B.; Vento, M. Analysis of lipid peroxidation biomarkers in extremely low gestational age neonate urines by UPLC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 4345–4356. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef]
- Ahsan, H. Diabetic retinopathy--biomolecules and multiple pathophysiology. Diabetes Metab. Syndr. 2014, 9, 51–54. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Das, A. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef] [Green Version]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Liu, A.; Chang, J.; Lin, Y.; Shen, Z.; Bernstein, P.S. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid. Res. 2010, 51, 3217–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Anderson, R.E. Dual roles of polyunsaturated fatty acids in retinal physiology and pathophysiology associated with retinal degeneration. Clin. Lipidol. 2009, 4, 7. [Google Scholar] [CrossRef]
- Suzumura, A.; Terao, R.; Kaneko, H. Protective Effects and Molecular Signaling of n-3 Fatty Acids on Oxidative Stress and Inflammation in Retinal Diseases. Antioxid. Basel 2020, 9, 920. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Omega-3 fatty acids in prevention of diabetic retinopathy. J. Pharm. Pharmacol. 2017, 69, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, Y.; Roose, N.; De Groef, L.; Fransen, M.; Moons, L.; Van Veldhoven, P.P.; Baes, M. Differential distribution of peroxisomal proteins points to specific roles of peroxisomes in the murine retina. Mol. Cell Biochem. 2019, 456, 53–62. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, W.C.; Hung, K.H.; Yang, D.M.; Cheng, Y.H.; Liao, Y.W.; Woung, L.C.; Tsai, C.Y.; Hsu, C.C.; Lin, T.C.; et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front. Aging Neurosci. 2014, 6, 191. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. Lond. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress—Nuclear factor κB—Apoptosis pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Li, W.; Jiang, D. Effect of resveratrol on Bcl-2 and VEGF expression in oxygen-induced retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2012, 49, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-related Macular Degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [CrossRef]
- Zhuang, P.; Shen, Y.; Lin, B.Q.; Zhang, W.Y.; Chiou, G.C. Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration(AMD). Eye Sci. 2011, 26, 23–29. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Wu, B.J.; Pan, W.H.; Zhang, X.M.; Liu, J.H.; Chen, M.M.; Chao, F.P.; Chao, H.M. Resveratrol mitigates rat retinal ischemic injury: The roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J. Ocul. Pharmacol. Ther. 2012, 29, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, M.; Kumar, V.; Singh, A.K.; Kashyap, M.P.; Khanna, V.K.; Siddiqui, M.A.; Pant, A.B. trans-Resveratrol protects ischemic PC12 Cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem. Neurosci. 2013, 4, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Retinal and choroidal neovascularization. J. Cell Physiol. 2000, 184, 301–310. [Google Scholar] [CrossRef]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal Nerve Fiber Layer and Ganglion Cell Complex Thicknesses Are Reduced in Children With Type 1 Diabetes With No Evidence of Vascular Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5355–5360. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y.; Tan, H.Y.; Li, S.; Wang, N.; Zhang, Y.; Feng, Y. Neuroprotective effect of He-Ying-Qing-Re formula on retinal ganglion cell in diabetic retinopathy. J. Ethnopharmacol. 2018, 214, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Galano, J.M.; Oger, C.; Leoncini, S.; Cortelazzo, A.; Ciccoli, L.; Durand, T.; Hayek, J.; Lee, J.C. Isoprostanoids in Clinical and Experimental Neurological Disease Models. Antioxidants 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Hu, J.; Du, S.; Chen, Y.; Wang, S.; Wu, Q. ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Mol. Vis. 2014, 20, 1109–1121. [Google Scholar]
- Ayalasomayajula, S.P.; Kompella, U.B. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003, 458, 283–289. [Google Scholar] [CrossRef]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–785S. [Google Scholar] [CrossRef] [Green Version]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys Acta 2015, 1852, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, L.; Palacios-Ortega, S.; Fernández-Quintela, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients 2019, 11, 961. [Google Scholar] [CrossRef] [Green Version]
- Al-Shabrawey, M.; Smith, S. Prediction of diabetic retinopathy: Role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010, 1, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangvarasittichai, O.; Tangvarasittichai, S. Oxidative Stress, Ocular Disease and Diabetes Retinopathy. Curr. Pharm Des. 2018, 24, 4726–4741. [Google Scholar] [CrossRef]
- Wang, M.H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef]
- Cheng, T.; Cao, W.; Wen, R.; Steinberg, R.H.; LaVail, M.M. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Müller cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 581–591. [Google Scholar]
- Kim, S.J.; Toma, H.S.; Barnett, J.M.; Penn, J.S. Ketorolac inhibits choroidal neovascularization by suppression of retinal VEGF. Exp. Eye Res. 2010, 91, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Schoenberger, S.D.; Kim, S.J.; Sheng, J.; Rezaei, K.A.; Lalezary, M.; Cherney, E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5906–5911. [Google Scholar] [CrossRef] [Green Version]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Kirchhof, B.; Koizumi, K.; Döhmen, S.; Adamis, A.P. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002, 16, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Sennlaub, F.; Valamanesh, F.; Vazquez-Tello, A.; El-Asrar, A.M.; Checchin, D.; Brault, S.; Gobeil, F.; Beauchamp, M.H.; Mwaikambo, B.; Courtois, Y.; et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 2003, 108, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [Green Version]
- Cracowski, J.L.; Camus, L.; Durand, T.; Devillier, P.; Guy, A.; Hardy, G.; Stanke-Labesque, F.; Rossi, J.C.; Bessard, G. Response of rat thoracic aorta to F(2)-isoprostane metabolites. J. Cardiovasc. Pharmacol. 2002, 39, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, F.; Qi, A.; Liu, L.; Zhang, J.; Xu, S.; Zhong, Q.; Chen, Y.; Zhang, C.Y.; Cai, C. Integration of ultra-high-pressure liquid chromatography-tandem mass spectrometry with machine learning for identifying fatty acid metabolite biomarkers of ischemic stroke. Chem. Commun. Camb. 2020, 56, 6656–6659. [Google Scholar] [CrossRef]
- Morrow, J.D.; Minton, T.A.; Mukundan, C.R.; Campbell, M.D.; Zackert, W.E.; Daniel, V.C.; Badr, K.F.; Blair, I.A.; Roberts, L.J. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994, 269, 4317–4326. [Google Scholar] [CrossRef]
- Longmire, A.W.; Roberts, L.J.; Morrow, J.D. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/endoperoxide receptor in humans and rats: Additional evidence for the existence of a unique isoprostane receptor. Prostaglandins 1994, 48, 247–256. [Google Scholar] [CrossRef]
- VanRollins, M.; Woltjer, R.L.; Yin, H.; Morrow, J.D.; Montine, T.J. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008, 49, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.J.; Montine, T.J.; Markesbery, W.R.; Tapper, A.R.; Hardy, P.; Chemtob, S.; Dettbarn, W.D.; Morrow, J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998, 273, 13605–13612. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.; Carrasco-Torres, R.; Amor, I.; Oger, C.; Galano, J.-M.; Durand, T.; Villegas Martínez, I.; Auvin, S.; Ferreres, F.; Gil-Izquierdo, Á. Antiepileptic drugs affect lipid oxidative markers- neuroprostanes and F2-dihomo-isoprostanes- in patients with epilepsy: Differences among first-, second-, and third-generation drugs by UHPLC-QqQ-MS/MS. RSC Adv. 2016, 6, 8. [Google Scholar] [CrossRef]
- Medina, S.; Miguel-Elízaga, I.D.; Oger, C.; Galano, J.M.; Durand, T.; Martínez-Villanueva, M.; Castillo, M.L.; Villegas-Martínez, I.; Ferreres, F.; Martínez-Hernández, P.; et al. Dihomo-isoprostanes-nonenzymatic metabolites of AdA-are higher in epileptic patients compared to healthy individuals by a new ultrahigh pressure liquid chromatography-triple quadrupole-tandem mass spectrometry method. Free Radic Biol. Med. 2015, 79, 154–163. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, L.A.; Medina, S.; Martínez-Hernández, P.; Oger, C.; Galano, J.M.; Durand, T.; Casas-Pina, T.; Ferreres, F.; Gil-Izquierdo, Á. Snapshot situation of oxidative degradation of the nervous system, kidney, and adrenal glands biomarkers-neuroprostane and dihomo-isoprostanes-urinary biomarkers from infancy to elderly adults. Redox Biol. 2017, 11, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Carrascosa-Marco, P.; Oger, C.; Vigor, C.; Galano, J.M.; Durand, T.; Baquero, M.; López-Nogueroles, M.; Vento, M.; García-Blanco, A.; et al. Validated analytical method to determine new salivary lipid peroxidation compounds as potential neurodegenerative biomarkers. J. Pharm. Biomed. Anal. 2019, 164, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Bautista, C.; Álvarez, L.; Durand, T.; Vigor, C.; Cuevas, A.; Baquero, M.; Vento, M.; Hervás, D.; Cháfer-Pericás, C. Clinical Utility of Plasma Lipid Peroxidation Biomarkers in Alzheimer’s Disease Differential Diagnosis. Antioxidants 2020, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003. [Google Scholar] [CrossRef]
- Early Photocoagulation for Diabetic Retinopathy: ETDRS Report Number 9. Ophthalmology 1991. [CrossRef]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Galano, J.M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Bartoli, M.; El-Remessy, A.B.; Ma, G.; Matragoon, S.; Lemtalsi, T.; Caldwell, R.W.; Caldwell, R.B. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3231–3238. [Google Scholar] [CrossRef]
- Davì, G.; Ciabattoni, G.; Consoli, A.; Mezzetti, A.; Falco, A.; Santarone, S.; Pennese, E.; Vitacolonna, E.; Bucciarelli, T.; Costantini, F.; et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation 1999, 99, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, L.K.; Bhaduri, G.; Bhattacharya, B. Biochemical scenario behind initiation of diabetic retinopathy in type 2 diabetes mellitus. Indian. J. Ophthalmol. 2018, 66, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Zagórski, Z. Lipid peroxidation in diabetic retinopathy. Ann. Univ. Mariae Curie Sklodowska Med. 2004, 59, 434–437. [Google Scholar] [PubMed]
- Van’t Erve, T.J.; Lih, F.B.; Jelsema, C.; Deterding, L.J.; Eling, T.E.; Mason, R.P.; Kadiiska, M.B. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic. Biol. Med. 2016, 95, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. 1), S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Liu, E.H.; Anggård, E.; Halliwell, B. F4-isoprostanes: A novel class of prostanoids formed during peroxidation of docosahexaenoic acid (DHA). Biochem. Biophys. Res. Commun. 1998, 242, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J.P.; Porter, N.A.; Moore, K.P.; Sheller, J.R.; Roberts, L.J. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. USA 2002, 99, 16713–16718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Torre, A.; Lee, Y.Y.; Oger, C.; Sangild, P.T.; Durand, T.; Lee, J.C.; Galano, J.M. Synthesis, discovery, and quantitation of dihomo-isofurans: Biomarkers for in vivo adrenic acid peroxidation. Angew. Chem. Int. Ed. Engl. 2014, 53, 6249–6252. [Google Scholar] [CrossRef]
- Song, W.L.; Lawson, J.A.; Reilly, D.; Rokach, J.; Chang, C.T.; Giasson, B.; FitzGerald, G.A. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J. Biol. Chem. 2008, 283, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Leung, H.H.; Ng, A.L.; Durand, T.; Kawasaki, R.; Oger, C.; Balas, L.; Galano, J.M.; Wong, I.Y.; Chung-Yung Lee, J. Increase in omega-6 and decrease in omega-3 polyunsaturated fatty acid oxidation elevates the risk of exudative AMD development in adults with Chinese diet. Free Radic. Biol. Med. 2019, 145, 349–356. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Cuevas, I.; Millán, I.; Asensi, M.; Vento, M.; Oger, C.; Galano, J.-M.; Durand, T.; Ortega, Á.L. Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene. Antioxidants 2021, 10, 168. https://doi.org/10.3390/antiox10020168
Torres-Cuevas I, Millán I, Asensi M, Vento M, Oger C, Galano J-M, Durand T, Ortega ÁL. Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene. Antioxidants. 2021; 10(2):168. https://doi.org/10.3390/antiox10020168
Chicago/Turabian StyleTorres-Cuevas, Isabel, Iván Millán, Miguel Asensi, Máximo Vento, Camille Oger, Jean-Marie Galano, Thierry Durand, and Ángel L. Ortega. 2021. "Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene" Antioxidants 10, no. 2: 168. https://doi.org/10.3390/antiox10020168
APA StyleTorres-Cuevas, I., Millán, I., Asensi, M., Vento, M., Oger, C., Galano, J. -M., Durand, T., & Ortega, Á. L. (2021). Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene. Antioxidants, 10(2), 168. https://doi.org/10.3390/antiox10020168