Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways
Abstract
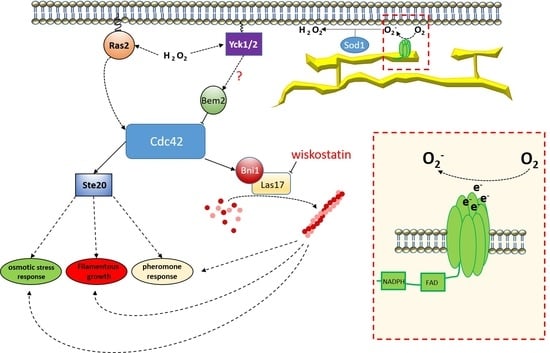
:1. Introduction
2. Material and Methods
2.1. Strains and Media
2.2. Cloning Experiments
2.3. DHE Measurements
2.4. Reporter Measurements
2.5. Spot Test Assay
2.6. Assays to Evaluate Filamentous/Invasive Growth
2.7. Immunoblot Analysis
2.8. Osmotic Stress and FM4-64 Staining
2.9. Pheromone Response Assay
2.10. Actin Morphology
2.11. Statistical Analysis
2.12. Gene Accession Numbers
3. Results
3.1. Establishment of an YNO1 Expression Reporter
3.2. Yno1p and the Osmotic Stress Response
3.3. Yno1p and the Pheromone Response
3.4. Evaluating the Role of Yno1p in Regulating Filamentous Growth
3.5. Yno1p, Actin Polymerization and Filamentous Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Breitenbach, M.; Rinnerthaler, M.; Weber, M.; Breitenbach-Koller, H.; Karl, T.; Cullen, P.; Basu, S.; Haskova, D.; Hasek, J. The defense and signaling role of NADPH oxidases in eukaryotic cells: Review. Wien. Med. Wochenschr. 2018, 168, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Royer-Pokora, B.; Kunkel, L.M.; Monaco, A.P.; Goff, S.C.; Newburger, P.E.; Baehner, R.L.; Cole, F.S.; Curnutte, J.T.; Orkin, S.H. Cloning the gene for an inherited human disorder—Chronic granulomatous disease—On the basis of its chromosomal location. Nature 1986, 322, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.C. The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol. Lett. 2017, 192, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological Defense Mechanisms the production by leukocytes of superoxide, a potential bactericidal agent. J. Immunol. 2014, 193, 5359–5362. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Buttner, S.; Laun, P.; Heeren, G.; Felder, T.K.; Klinger, H.; Weinberger, M.; Stolze, K.; Grousl, T.; Hasek, J.; et al. Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygen species generation, apoptosis, and actin cable formation in yeast. Proc. Natl. Acad. Sci. USA 2012, 109, 8658–8663. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.C.P.; Gleason, J.E.; Sanchez, H.; Schatzman, S.S.; Culbertson, E.M.; Johnson, C.J.; McNees, C.A.; Coelho, C.; Nett, J.E.; Andes, D.R.; et al. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017, 13. [Google Scholar] [CrossRef]
- Hajjar, C.; Cherrier, M.V.; Mirandela, G.D.; Petit-Hartlein, I.; Stasia, M.J.; Fontecilla-Camps, J.C.; Fieschi, F.; Dupuy, J. The NOX Family of Proteins Is Also Present in Bacteria. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddi, A.R.; Culotta, V.C. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 2013, 152, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.F.; Papanayotou, I.; Davis, N.G. The yeast kinase Yck2 has a tripartite palmitoylation signal. Mol. Biol. Cell 2011, 22, 2702–2715. [Google Scholar] [CrossRef]
- Babu, P.; Bryan, J.D.; Panek, H.R.; Jordan, S.L.; Forbrich, B.M.; Kelley, S.C.; Colvin, R.T.; Robinson, L.C. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J. Cell Sci. 2002, 115, 4957–4968. [Google Scholar] [CrossRef] [Green Version]
- Snowdon, C.; Johnston, M. A novel role for yeast casein kinases in glucose sensing and signaling. Mol. Biol. Cell 2016, 27, 3369–3375. [Google Scholar] [CrossRef]
- Alvaro, C.G.; O’Donnell, A.F.; Prosser, D.C.; Augustine, A.A.; Goldman, A.; Brodsky, J.L.; Cyert, M.S.; Wendland, B.; Thorner, J. Specific alpha-arrestins negatively regulate Saccharomyces cerevisiae pheromone response by down-modulating the G-protein-coupled receptor Ste2. Mol. Cell Biol. 2014, 34, 2660–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, D.J.; Mitchell, D.A.; Dong, X.W.; Deschenes, R.J. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell Biol. 1999, 19, 6775–6787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Johnston, M. Two glucose-sensing pathways converge on Rgt1 to regulate expression of glucose transporter genes in Saccharomyces cerevisiae. J. Biol Chem. 2006, 281, 26144–26149. [Google Scholar] [CrossRef] [Green Version]
- Leadsham, J.E.; Sanders, G.; Giannaki, S.; Bastow, E.L.; Hutton, R.; Naeimi, W.R.; Breitenbach, M.; Gourlay, C.W. Loss of Cytochrome c Oxidase Promotes RAS-Dependent ROS Production from the ER Resident NADPH Oxidase, Yno1p, in Yeast. Cell Metab. 2013, 18, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Gale, C.A.; Bendel, C.M.; McClellan, M.; Hauser, M.; Becker, J.M.; Berman, J.; Hostetter, M.K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 1998, 279, 1355–1358. [Google Scholar] [CrossRef]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, C.J.; Ljungdahl, P.O.; Styles, C.A.; Fink, G.R. Unipolar Cell Divisions in the Yeast Saccharomyces—Cerevisiae Lead to Filamentous Growth—Regulation by Starvation and Ras. Cell 1992, 68, 1077–1090. [Google Scholar] [CrossRef]
- Kron, S.J.; Styles, C.A.; Fink, G.R. Symmetric cell division in pseudohyphae of the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 1994, 5, 1003–1022. [Google Scholar] [CrossRef]
- Cali, B.M.; Doyle, T.C.; Botstein, D.; Fink, G.R. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 1998, 9, 1873–1889. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Kohler, T.; Braus, G.H.; Mosch, H.U. Asymmetrically localized Bud8p and Bud9p proteins control yeast cell polarity and development. EMBO J. 2000, 19, 6686–6696. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Fink, G.R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: Mating and invasive growth. Genes Dev. 1994, 8, 2974–2985. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.S.; Fink, G.R. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 1998, 95, 13783–13787. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.C.; Heitman, J. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 1997, 16, 7008–7018. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Heitman, J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell Biol. 1999, 19, 4874–4887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, T.M.; Mitchell, A.P. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell Biol. 2003, 23, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, P.J.; Sprague, G.F. The Regulation of Filamentous Growth in Yeast. Genetics 2012, 190, 23–49. [Google Scholar] [CrossRef]
- Cullen, P.J.; Sabbagh, W.; Graham, E.; Irick, M.M.; van Olden, E.K.; Neal, C.; Delrow, J.; Bardwell, L.; Sprague, G.F. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Gene Dev. 2004, 18, 1695–1708. [Google Scholar] [CrossRef] [Green Version]
- Peter, M.; Neiman, A.M.; Park, H.O.; vanLohuizen, M.; Herskowitz, I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 1996, 15, 7046–7059. [Google Scholar] [CrossRef]
- Gancedo, J.M. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 107–123. [Google Scholar] [CrossRef]
- Mosch, H.U.; Roberts, R.L.; Fink, G.R. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 5352–5356. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.E.; Thorner, J. Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2007, 1773, 1311–1340. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.G.; Swamy, S.R.; Pon, L.A. The life cycle of actin patches in mating yeast. J. Cell Sci. 2001, 114, 1505–1513. [Google Scholar] [PubMed]
- Severin, F.F.; Hyman, A.A. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 2002, 12, R233–235. [Google Scholar] [CrossRef] [Green Version]
- Saito, H. Regulation of cross-talk in yeast MAPK signaling pathways. Curr. Opin. Microbiol. 2010, 13, 677–683. [Google Scholar] [CrossRef]
- Nishimura, A.; Yamamoto, K.; Oyama, M.; Kozuka-Hata, H.; Saito, H.; Tatebayashi, K. Scaffold Protein Ahk1, Which Associates with Hkr1, Sho1, Ste11, and Pbs2, Inhibits Cross Talk Signaling from the Hkr1 Osmosensor to the Kss1 Mitogen-Activated Protein Kinase. Mol. Cell Biol. 2016, 36, 1109–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitoniak, A.; Birkaya, B.; Dionne, H.M.; Vadaie, N.; Cullen, P.J. The Signaling Mucins Msb2 and Hkr1 Differentially Regulate the Filamentation Mitogen-activated Protein Kinase Pathway and Contribute to a Multimodal Response. Mol. Biol. Cell 2009, 20, 3101–3114. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tatebayashi, K.; Nishimura, A.; Yamamoto, K.; Yang, H.Y.; Saito, H. Yeast Osmosensors Hkr1 and Msb2 Activate the Hog1 MAPK Cascade by Different Mechanisms. Sci. Signal. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Tatebayashi, K.; Tanaka, K.; Yang, H.Y.; Yamamoto, K.; Matsushita, Y.; Tomida, T.; Imai, M.; Saito, H. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 2007, 26, 3521–3533. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, S. Osmotic stress signaling and osmoadaptation in Yeasts. Microbiol. Mol. Biol. R. 2002, 66, 300–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, P.T.; Oliveira, J.; Pais, P.; Antunes, M.; Palma, M.; Cavalheiro, M.; Galocha, M.; Godinho, C.P.; Martins, L.C.; Bourbon, N.; et al. YEASTRACT plus: A portal for cross-species comparative genomics of transcription regulation in yeasts. Nucleic Acids Res. 2020, 48, D642–D649. [Google Scholar] [CrossRef] [PubMed]
- Isgandarova, S.; Jones, L.; Forsberg, D.; Loncar, A.; Dawson, J.; Tedrick, K.; Eitzen, G. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J. Biol. Chem. 2007, 282, 30466–30475. [Google Scholar] [CrossRef] [Green Version]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. BBA-Mol. Cell Res. 2009, 1793, 650–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.C.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Van Dyk, D.; Pretorius, I.S.; Bauer, F.F. Mss11p is a central element of the regulatory network that controls FLO11 expression and invasive growth in Saccharomyces cerevisiae. Genetics 2005, 169, 91–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinger, H.; Rinnerthaler, M.; Lam, Y.T.; Laun, P.; Heeren, G.; Klocker, A.; Simon-Nobbe, B.; Dickinson, J.R.; Dawes, I.W.; Breitenbach, M. Quantitation of (a)symmetric inheritance of functional and of oxidatively damaged mitochondrial aconitase in the cell division of old yeast mother cells. Exp. Gerontol. 2010, 45, 533–542. [Google Scholar] [CrossRef]
- Streubel, M.K.; Bischof, J.; Weiss, R.; Duschl, J.; Liedl, W.; Wimmer, H.; Breitenbach, M.; Weber, M.; Geltinger, F.; Richter, K.; et al. Behead and live long or the tale of cathepsin L. Yeast 2018, 35, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Cullen, P.J.; Sprague, G.F. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 13619–13624. [Google Scholar] [CrossRef] [Green Version]
- Zupan, J.; Raspor, P. Quantitative agar-invasion assay. J. Microbiol. Methods 2008, 73, 100–104. [Google Scholar] [CrossRef]
- Basu, S.; Vadaie, N.; Prabhakar, A.; Li, B.; Adhikari, H.; Pitoniak, A.; Chow, J.; Chavel, C.A.; Cullen, P.J. Spatial landmarks regulate a Cdc42-dependent MAPK pathway to control differentiation and the response to positional compromise. Proc. Natl. Acad. Sci. USA 2016, 113, E2019–2028. [Google Scholar] [CrossRef] [Green Version]
- Michaillat, L.; Mayer, A. Identification of Genes Affecting Vacuole Membrane Fragmentation in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e54160. [Google Scholar] [CrossRef] [Green Version]
- Heeren, G.; Rinnerthaler, M.; Laun, P.; von Seyerl, P.; Kossler, S.; Klinger, H.; Hager, M.; Bogengruber, E.; Jarolim, S.; Simon-Nobbe, B.; et al. The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1. Aging-Us 2009, 1, 622–636. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.M.; MacIver, F.H.; Dawes, I.W. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997, 410, 219–222. [Google Scholar] [CrossRef]
- Pyatrikas, D.V.; Fedoseeva, I.V.; Varakina, N.N.; Rusaleva, T.M.; Stepanov, A.V.; Fedyaeva, A.V.; Borovskii, G.B.; Rikhvanov, E.G. Relation between cell death progression, reactive oxygen species production and mitochondrial membrane potential in fermenting Saccharomyces cerevisiae cells under heat-shockconditions. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.C.; Wosniak, J., Jr.; Pescatore, L.A.; Bertoline, M.A.; Liberman, M.; Laurindo, F.R.; Santos, C.X. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am. J. Physiol. Cell Physiol. 2007, 292, C413–422. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Nazarewicz, R.; Panov, A.; Harrison, D.G.; Dikalova, A. Crosstalk Between Mitochondrial ROS and NADPH Oxidases in Cardiovascular and Degenerative Diseases: Application of Mitochondria-Targeted Antioxidants. Free Radic. Bio. Med. 2011, 51, S85–S86. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (Ros) and Ros-Induced Ros Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Yi, D.G.; Hong, S.; Huh, W.K. Mitochondrial dysfunction reduces yeast replicative lifespan by elevating RAS-dependent ROS production by the ER-localized NADPH oxidase Yno1. PLoS ONE 2018, 13, e0198619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateus, C.; Avery, S.V. Destabilized green fluorescent protein for monitoring dynamic changes in yeast gene expression with flow cytometry. Yeast 2000, 16, 1313–1323. [Google Scholar] [CrossRef]
- Peterson, J.R.; Bickford, L.C.; Morgan, D.; Kim, A.S.; Ouerfelli, O.; Kirschner, M.W.; Rosen, M.K. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat. Struct. Mol. Biol. 2004, 11, 747–755. [Google Scholar] [CrossRef]
- Madania, A.; Dumoulin, P.; Grava, S.; Kitamoto, H.; Scharer-Brodbeck, C.; Soulard, A.; Moreau, V.; Winsor, B. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell 1999, 10, 3521–3538. [Google Scholar] [CrossRef] [Green Version]
- Aspenstrom, P. The verprolin family of proteins: Regulators of cell morphogenesis and endocytosis. FEBS Lett. 2005, 579, 5253–5259. [Google Scholar] [CrossRef] [Green Version]
- Tyler, J.J.; Allwood, E.G.; Ayscough, K.R. WASP family proteins, more than Arp2/3 activators. Biochem. Soc. Trans. 2016, 44, 1339–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raitt, D.C.; Posas, F.; Saito, H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000, 19, 4623–4631. [Google Scholar] [CrossRef]
- Leberer, E.; Dignard, D.; Harcus, D.; Thomas, D.Y.; Whiteway, M. The Protein-Kinase Homolog Ste20p Is Required to Link the Yeast Pheromone Response G-Protein Beta-Gamma Subunits to Downstream Signaling Components. EMBO J. 1992, 11, 4815–4824. [Google Scholar] [CrossRef]
- Huh, G.H.; Damsz, B.; Matsumoto, T.K.; Reddy, M.P.; Rus, A.M.; Ibeas, J.I.; Narasimhan, M.L.; Bressan, R.A.; Hasegawa, P.M. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002, 29, 649–659. [Google Scholar] [CrossRef]
- Wadskog, I.; Maldener, C.; Proksch, A.; Madeo, F.; Adler, L. Yeast lacking the SRO7/SOP1-encoded tumor suppressor homologue show increased susceptibility to apoptosis-like cell death on exposure to NaCl stress. Mol. Biol. Cell 2004, 15, 1436–1444. [Google Scholar] [CrossRef] [Green Version]
- Rep, M.; Reiser, V.; Gartner, U.; Thevelein, J.M.; Hohmann, S.; Ammerer, G.; Ruis, H. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell Biol. 1999, 19, 5474–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorner, W.; Durchschlag, E.; Martinez-Pastor, M.T.; Estruch, F.; Ammerer, G.; Hamilton, B.; Ruis, H.; Schuller, C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Gene Dev. 1998, 12, 586–597. [Google Scholar] [CrossRef]
- Berry, D.B.; Gasch, A.P. Stress-activated Genomic Expression Changes Serve a Preparative Role for Impending Stress in Yeast. Mol. Biol. Cell 2008, 19, 4580–4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miermont, A.; Waharte, F.; Hu, S.Q.; McClean, M.N.; Bottani, S.; Leon, S.; Hersen, P. Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc. Natl. Acad. Sci. USA 2013, 110, 5725–5730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vida, T.A.; Emr, S.D. A New Vital Stain for Visualizing Vacuolar Membrane Dynamics and Endocytosis in Yeast. J. Cell Biol. 1995, 128, 779–792. [Google Scholar] [CrossRef]
- Dickinson, J.R. ‘Fusel’ alcohols induce hyphal-like extensions and pseudohyphal formation in yeasts. Microbiology 1996, 142, 1391–1397. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.C.; Cutler, N.S.; Heitman, J. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 2000, 11, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, H.; Cullen, P.J. Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Madhani, H.D.; Galitski, T.; Lander, E.S.; Fink, G.R. Effectors of a developmental mitogen-activated protein kinase cascade revealed by expression signatures of signaling mutants. Proc. Natl. Acad. Sci. USA 1999, 96, 12530–12535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palecek, S.P.; Parikh, A.S.; Kron, S.J. Genetic analysis reveals that FLO11 upregulation and cell polarization independently regulate invasive growth in Saccharomyces cerevisiae. Genetics 2000, 156, 1005–1023. [Google Scholar] [PubMed]
- Purevdorj-Gage, B.; Orr, M.E.; Stoodley, P.; Sheehan, K.B.; Hyman, L.E. The role of FLO11 in Saccharomyces cerevisiae biofilm development in a laboratory based flow-cell system. FEMS Yeast Res. 2007, 7, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Cullen, P.J. The plate-washing assay: A simple test for filamentous growth in budding yeast. Cold Spring Harb. Protoc. 2015, 2015, 168–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, J.; Hekimi, S. When a theory of aging ages badly. Cell Mol. Life Sci. 2010, 67, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Cheng, X.X.; Yu, Q.L.; Qian, K.F.; Ding, X.H.; Liu, R.M.; Zhang, B.; Xing, L.J.; Li, M.C. Aft2, a Novel Transcription Regulator, Is Required for Iron Metabolism, Oxidative Stress, Surface Adhesion and Hyphal Development in Candida albicans. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, L.; Liang, S.; Zhang, P.; Kang, R.; Zhang, M.; Wang, M.; Chen, L.; Yuan, H.; Ding, S.; et al. FpDep1, a component of Rpd3L histone deacetylase complex, is important for vegetative development, ROS accumulation, and pathogenesis in Fusarium pseudograminearum. Fungal Genet. Biol. 2020, 135, 103299. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Znaidi, S.; Lagage, V.; Cabral, V.; Schoenherr, F.; LeibundGut-Landmann, S.; d’Enfert, C.; Bachellier-Bassi, S. The two-component response regulator Skn7 belongs to a network of transcription factors regulating morphogenesis in Candida albicans and independently limits morphogenesis-induced ROS accumulation. Mol. Microbiol. 2017, 106, 157–182. [Google Scholar] [CrossRef] [Green Version]
- Breitenbach, M.; Weber, M.; Rinnerthaler, M.; Karl, T.; Breitenbach-Koller, L. Oxidative Stress in Fungi: Its Function in Signal Transduction, Interaction with Plant Hosts, and Lignocellulose Degradation. Biomolecules 2015, 5, 318–342. [Google Scholar] [CrossRef] [Green Version]
- Malagnac, F.; Lalucque, H.; Lepere, G.; Silar, P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet. Biol. 2004, 41, 982–997. [Google Scholar] [CrossRef]
- Lara-Ortiz, T.; Riveros-Rosas, H.; Aguirre, J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 2003, 50, 1241–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Dominguez, N.; Alvarez-Delfin, K.; Hansberg, W.; Aguirre, J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot. Cell 2008, 7, 1352–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayano, Y.; Tanaka, A.; Akano, F.; Scott, B.; Takemoto, D. Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloe festucae. Fungal Genet. Biol. 2013, 56, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Baryshnikova, A.; Bellay, J.; Kim, Y.; Spear, E.D.; Sevier, C.S.; Ding, H.; Koh, J.L.; Toufighi, K.; Mostafavi, S.; et al. The genetic landscape of a cell. Science 2010, 327, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, M.; VanderSluis, B.; Koch, E.N.; Baryshnikova, A.; Pons, C.; Tan, G.; Wang, W.; Usaj, M.; Hanchard, J.; Lee, S.D.; et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 2016, 353. [Google Scholar] [CrossRef]
- Sharifpoor, S.; van Dyk, D.; Costanzo, M.; Baryshnikova, A.; Friesen, H.; Douglas, A.C.; Youn, J.Y.; VanderSluis, B.; Myers, C.L.; Papp, B.; et al. Functional wiring of the yeast kinome revealed by global analysis of genetic network motifs. Genome Res. 2012, 22, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, P.; Rincon, S.A. Rho GTPases: Regulation of cell polarity and growth in yeasts. Biochem. J. 2010, 426, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, M.; Klebl, B.M.; Tong, A.H.Y.; Webb, B.A.; Leeuw, T.; Leberer, E.; Whiteway, M.; Thomas, D.Y.; Boone, C. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 2000, 148, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedrick, K.; Trischuk, T.; Lehner, R.; Eitzen, G. Enhanced membrane fusion in sterol-enriched vacuoles bypasses the Vrp1p requirement. Mol. Biol. Cell 2004, 15, 4609–4621. [Google Scholar] [CrossRef] [Green Version]
- Montllor-Albalate, C.; Colin, A.E.; Chandrasekharan, B.; Bolaji, N.; Andersen, J.L.; Outten, F.W.; Reddi, A.R. Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol. 2019, 21. [Google Scholar] [CrossRef]
- Martiniere, A.; Fiche, J.B.; Smokvarska, M.; Mari, S.; Alcon, C.; Dumont, X.; Hematy, K.; Jaillais, Y.; Nollmann, M.; Maurel, C. Osmotic Stress Activates Two Reactive Oxygen Species Pathways with Distinct Effects on Protein Nanodomains and Diffusion. Plant Physiol. 2019, 179, 1581–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, M.E.; Sirotkin, V.; Haarer, B.; Kakhniashvili, D.; Amberg, D.C. Diverse protective roles of the actin cytoskeleton during oxidative stress. Cytoskeleton 2011, 68, 340–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Gutierrez, D.; Eisenberg, T.; Buttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, G.G.; Tan, S.X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpova, T.S.; McNally, J.G.; Moltz, S.L.; Cooper, J.A. Assembly and function of the actin cytoskeleton of yeast: Relationships between cables and patches. J. Cell Biol. 1998, 142, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Smith, K.W.; Gustin, M.C. Osmotic stress and the yeast cytoskeleton: Phenotype-specific suppression of an actin mutation. J. Cell Biol. 1992, 118, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lechler, T.; Jonsdottir, G.A.; Klee, S.K.; Pellman, D.; Li, R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J. Cell Biol. 2001, 155, 261–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Basu, S.; González, B.; Greslehner, G.P.; Singer, S.; Haskova, D.; Hasek, J.; Breitenbach, M.; W.Gourlay, C.; Cullen, P.J.; et al. Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways. Antioxidants 2021, 10, 322. https://doi.org/10.3390/antiox10020322
Weber M, Basu S, González B, Greslehner GP, Singer S, Haskova D, Hasek J, Breitenbach M, W.Gourlay C, Cullen PJ, et al. Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways. Antioxidants. 2021; 10(2):322. https://doi.org/10.3390/antiox10020322
Chicago/Turabian StyleWeber, Manuela, Sukanya Basu, Beatriz González, Gregor P. Greslehner, Stefanie Singer, Danusa Haskova, Jiri Hasek, Michael Breitenbach, Campbell W.Gourlay, Paul J. Cullen, and et al. 2021. "Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways" Antioxidants 10, no. 2: 322. https://doi.org/10.3390/antiox10020322
APA StyleWeber, M., Basu, S., González, B., Greslehner, G. P., Singer, S., Haskova, D., Hasek, J., Breitenbach, M., W.Gourlay, C., Cullen, P. J., & Rinnerthaler, M. (2021). Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways. Antioxidants, 10(2), 322. https://doi.org/10.3390/antiox10020322