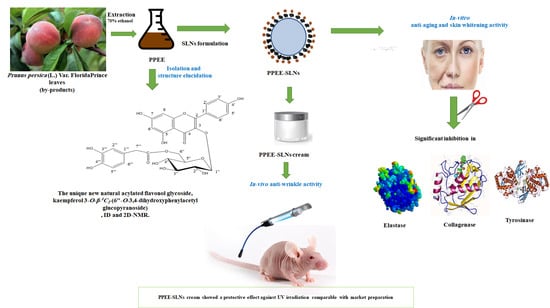
A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare
Abstract
:1. Introduction
2. Material and Methods
2.1. General
2.2. Plant Materials
2.3. Preparation of Plant Extract
2.4. Determination of Total Polyphenols Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Fractionation of PPEE and Isolation of Compound 1
2.7. Identification of kaempferol 3-O-β-4C1-(6”-O-3,4-dihydroxyphenylacetyl glucopyranoside) (1)
2.8. In-Vitro Studies
2.8.1. Evaluation of Cytotoxicity in Human Keratinocytes
2.8.2. In-Vitro Antioxidant Assays
2.8.3. Estimation of the Anti-Elastase, Anti-Collagenase, and Anti-Tyrosinase Activities
2.9. Formulation and Characterization of PPEE-SLNs
2.10. Preparation of PPEE Topical Cream Formulae
2.10.1. Formulation of PPEE-SLNs Cream
2.10.2. Evaluation Parameters of PPEE-SLNs Cream
2.11. In-Vivo Anti-Wrinkle Study of PPEE-SLNs Cream
2.11.1. Experimental Animals
2.11.2. Wrinkle Score Measurement
2.11.3. Histological Evaluation
2.11.4. Superoxide Dismutase (SOD) Activity
2.12. Statistical Analysis
3. Results
3.1. Total Phenolic and Flavonoid Contents
3.2. Isolation and Structure Elucidation of kaempferol 3-O-β-4C1-(6″-O-3,4-dihydroxyphenylacetyl glucopyranoside), Compound 1
3.3. In-Vitro Studies
3.3.1. Evaluation of Cytotoxicity in Human Keratinocytes
3.3.2. In-Vitro Antioxidant Assays
3.3.3. Estimation of the Anti-Elastase, Anti-Collagenase, and Anti-Tyrosinase Activities
3.4. Evaluation of PPEE-SLNs and PPEE-SLNs Cream
3.5. In-Vivo Studies
3.5.1. Anti-Wrinkle Activity Exerted by PPEE-SLNs Cream
3.5.2. Histological Evaluation
3.5.3. Estimation of Superoxide Dismutase (SOD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Kim, J.E.; Choi, S.I.; Lee, H.R.; Lee, Y.J.; Jang, M.J.; Son, H.J.; Lee, H.S.; Oh, C.H.; Kim, B.H. UV radiation-induced skin aging in hairless mice is effectively prevented by oral intake of sea buckthorn (Hippophae rhamnoides L.) fruit blend for 6 weeks through MMP suppression and increase of SOD activity. Int. J. Mol. Med. 2012, 30, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, C. Molecular mechanisms of skin photoaging and plant inhibitors. Int. J. Green Pharm. 2017, 11, 3268. [Google Scholar]
- Kang, M.; Park, S.-H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Nho, Y.H.; Lee, S.; Han, B.S.; Cho, J.Y.; Lee, J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotechnol. Biochem. 2018, 82, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmiaty, Y.; Saputri, F.C.; Hanafi, M.; Prastiwi, R.; Elya, B. Anti-elastase, anti-tyrosinase and anti-oxidant of Rubus fraxinifolius Stem Methanolic Extract. Pharmacogn. J. 2020, 12, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Rasul, A.; Akhtar, N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non-invasive biophysical techniques. DARU J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 344. [Google Scholar]
- Salavkar, S.M.; Tamanekar, R.A.; Athawale, R.B. Antioxidants in skin ageing—Future of dermatology. Int. J. Green Pharm. 2011, 5, 161–168. [Google Scholar]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Choubey, A.; Gilhotra, R.; Singh, S.K.; Garg, G. Formulation and characterization of nanomedicine (solid lipid nanoparticle) associate with the extract of Pterospermum acerifolium for the screening of neurochemicals and neuroendocrine effects. Asian J. Neurosurg. 2017, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Vaugban, J.G.; Geissler, C.A. The New Oxford Book of Food Plants, 2nd ed.; Oxford University Press: New York, NY, USA, 1999; pp. 172–179. [Google Scholar]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Soulef, S.; Seddik, K.; Nozha, M.; Smain, A.; Saliha, D.; Hosni, K. Phytochemical screening and in vivo and in vitro evaluation antioxidant capacity of Fargaria ananassa, Prunus armeniaca and Prunus persica fruits growing in Algeria. Prog. Nutr. 2020, 22, 236–252. [Google Scholar]
- Stierlin, E.; Azoulay, S.; Massi, L.; Fernandez, X.; Michel, T. Cosmetic potentials of Prunus domestica L. leaves. J. Sci. Food Agric. 2018, 98, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Vascular Plants; Cambridge University Press: Cambridge, MA, USA, 1997; ISBN 0521414210. [Google Scholar]
- Benmehdi, H.; Fellah, K.; Amrouche, A.; Memmou, F.; Malainine, H.; Dalile, H.; Siata, W. Phytochemical study, antioxidant activity and kinetic behaviour of flavonoids fractions isolated from Prunus persica L. Leaves. Asian J. Chem. 2017, 29, 13. [Google Scholar] [CrossRef]
- Gilani, A.H.; Aziz, N.; Ali, S.M.; Saeed, M. Pharmacological basis for the use of peach leaves in constipation. J. Ethnopharmacol. 2000, 73, 87–93. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, S.; Sharma, M.; Upadhyay, N.; Ahmed, Z.; Mahindroo, N. Anti-diabetic, anti-oxidant and anti-adipogenic potential of quercetin rich ethyl acetate fraction of Prunus persica. Pharmacogn. J. 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Mokrani, A.; Cluzet, S.; Madani, K.; Pakina, E.; Gadzhikurbanov, A.; Mesnil, M.; Monvoisin, A.; Richard, T. HPLC-DAD-MS/MS profiling of phenolics from different varieties of peach leaves and evaluation of their antioxidant activity: A comparative study. Int. J. Mass Spectrom. 2019, 445, 116192. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Nalbantsoy, A.; Yalcin, H.T.; Yesil-Celiktas, O. Cytotoxic, antimicrobial and nitric oxide inhibitory activities of supercritical carbon dioxide extracted Prunus persica leaves. Mol. Biol. Rep. 2020, 47, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, C.; Gupta, D.; Deb, L.; Debnath, S.; Dutta, A.S. Effect of leave extract of Prunus persica Linn on acute inflammation in rats. Res. J. Pharmacogn. Phytochem. 2011, 3, 38–40. [Google Scholar]
- Kwak, C.S.; Yang, J.; Shin, C.-Y.; Chung, J.H. Topical or oral treatment of peach flower extract attenuates UV-induced epidermal thickening, matrix metalloproteinase-13 expression and pro-inflammatory cytokine production in hairless mice skin. Nutr. Res. Pract. 2018, 12, 29. [Google Scholar] [CrossRef]
- Raturi, R.; Sati, S.C.; Badoni, P.P.; Singh, H.; Sati, M.D. Chemical constituents of Prunus persica stem bark. J. Sci. Res. 2012, 4, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Backheet, E.Y.; Farag, S.F.; Ahmed, A.S.; Sayed, H.M. Flavonoids and cyanogenic glycosides from the leaves and stem bark of Prunus persica (L.) Batsch (Meet Ghamr) peach local cultivar in Assiut region. Bull. Pharm. Sci. Assiut 2003, 26, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Upyr, T.V.; Jelev, I.S.; Lenchyk, L.V.; Komisarenko, M.A.; Abderrahim, A.; Poghosyan, O.G.; Dimova, G.I.; Yeromina, H.O. Study of Biologically Active Compounds in Prunus persica Leaves Extract. Res. J. Pharm. Technol. 2019, 12, 3273. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, H.; Shin, H.; Jeong, H.; Kim, J.; Kim, D. Cosmetic effects of Prunus padus bark extract. Korean J. Chem. Eng. 2014, 31, 2280–2285. [Google Scholar] [CrossRef]
- Sachdeva, M.K.; Katyal, T. Abatement of detrimental effects of photoaging by Prunus amygdalus skin extract. Int. J. Curr. Pharm. Res. 2011, 3, 57–59. [Google Scholar]
- Sile, I.; Videja, M.; Makrecka-Kuka, M.; Tirzite, D.; Pajuste, K.; Shubin, K.; Krizhanovska, V.; Grinberga, S.; Pugovics, O.; Dambrova, M. Chemical composition of Prunus padus L. flower extract and its anti-inflammatory activities in primary bone marrow-derived macrophages. J. Ethnopharmacol. 2020, 268, 113678. [Google Scholar] [CrossRef]
- Han, S.; Park, K.-K.; Chung, W.-Y.; Lee, S.K.; Kim, J.; Hwang, J.-K. Anti-photoaging effects of 2-methoxy-5-(2-methyl propyl) pyrazine isolated from peach (Prunus persica (L.) Batsch). Food Sci. Biotechnol. 2010, 19, 1667–1671. [Google Scholar] [CrossRef]
- Lee, J.-Y.; An, B.-J. Whitening and anti-wrinkle effects of Prunus persica Flos. J. Appl. Biol. Chem. 2010, 53, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-M.; Kim, K.-H.; Kim, Y.-S.; Koh, J.-H.; Lee, K.-H.; Yook, H.-S. A study on the development of cosmetic materials using unripe peaches seed extracts. J. Korean Soc. Food Sci. Nutr. 2012, 41, 110–115. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica by-products: A source of minerals, phenols and volatile compounds. Sci. Hortic. 2020, 261, 109016. [Google Scholar] [CrossRef]
- de Vargas, E.F.; Jablonski, A.; Flôres, S.H.; de Rios, A.O. Waste from peach (Prunus persica) processing used for optimisation of carotenoids ethanolic extraction. Int. J. Food Sci. Technol. 2017, 52, 757–762. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, E.S.; Nawwar, M.A.M.; Mostafa, D.A.; Ragab, M.F.; Swilam, N. Karafsin, a unique mono-acylated flavonoid apiofurnoside from the leaves of Apium graveolens var. secalinum Alef: In vitro and in vivo anti-inflammatory assessment. Ind. Crops Prod. 2020, 158, 112901. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimi Telforschung 1996, 46, 1086–1089. [Google Scholar]
- Yardpiroon, B.; Aphidech, S.; Prasong, S. Phytochemical and biological activities of the wild grape fruit extracts using different solvents. J. Pharm. Res. Int. 2014, 4, 23–36. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mostafa, E.; Fayed, M.A.A.; Radwan, R.A.; Bakr, R.O. Centaurea pumilio L. extract and nanoparticles: A candidate for healthy skin. Colloids Surf. B Biointerfaces 2019, 182, 110350. [Google Scholar] [CrossRef]
- Mahawar, V.; Patidar, K.; Joshi, N. Development and evaluation of herbal antiaging cream formulation containing Annona squamosa leaf extract. Asian J. Pharm. Clin. Res. 2019, 12, 210–214. [Google Scholar] [CrossRef]
- Matangi, S.P.; Mamidi, S.A.; Raghavamma, S.T.V.; Nadendla, R.R. Formulation and evaluation of anti aging poly herbal cream. Skin 2014, 5, 6. [Google Scholar]
- Sekar, M.; Sivalinggam, P.; Mahmad, A. Formulation and evaluation of novel antiaging cream containing rambutan fruits extract. Int. J. Pharm. Sci. Res. 2017, 8, 1056. [Google Scholar]
- Bissett, D.; Hannonand, D.; Orr, T. An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem. Photobiol. 1987, 46, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.; Elenistas, R.; Jaworsky, C.; Johnson, B. Lever’s Histopathology of the Skin, 8th ed.; Lippincott-Williams and Wilkins: Philadelphia, PA, USA, 1997. [Google Scholar]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(phenylamino)-carbonyl]-3, 4-tetrazolium}-bis (4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xanthine–xanthine oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.; Ayoub, N.; El-Raey, M.; Zaghloul, S.; Hashem, A.; Mostafa, E.; Eldahshan, O.; Lindequist, U.; Linscheid, M.W. Acylated flavonol diglucosides from Ammania auriculata. Z. Nat. C 2015, 70, 39–43. [Google Scholar] [CrossRef]
- Fellah, K.; Amrouche, A.; Benmehdi, H.; Memmou, F. Phenolic profile, antioxidants and kinetic properties of flavonoids and Tannins Fractions isolated from Prunus persica L. leaves growing in Southwest Algeria. Res. J. Pharm. Technol. 2019, 12, 4365–4372. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, L.; Zhang, N.; Wang, C.; Wu, W.; Mehmood, A.; Zhang, L.; Ji, B.; Zhou, F. Essential oil and juice from bergamot and sweet orange improve Acne vulgaris caused by excessive androgen secretion. Mediat. Inflamm. 2020. [Google Scholar] [CrossRef]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altinyazar, C.; Koca, R.; Tekin, N.S. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 763–767. [Google Scholar] [CrossRef]
- Veerasophon, J.; Sripalakit, P.; Saraphanchotiwitthaya, A. Formulation of anti-acne concealer containing cinnamon oil with antimicrobial activity against Propionibacterium acnes. J. Adv. Pharm. Technol. Res. 2020, 11, 53–58. [Google Scholar] [CrossRef]
- Isaac, V.L.B.; Chiari, B.G.; Miglioli, K.; Moreira, R.; Oliveira, J.R.S.; Salgado, H.; Relkin, P.; Correa, M.A.; Salgado, A.; Ribeiro, H.M. Development of a topical formulation containing S. Lutea extract: Stability, in vitro studies and cutaneous permeation. J. Appl. Pharm. Sci. 2012, 23, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kurniadi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Pimple, B.P.; Badole, S.L. Polyphenols: A remedy for skin wrinkles. In Polyphenols in Human Health and Disease. Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 861–869. ISBN 9780123984562. [Google Scholar]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement. Altern. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeta, G.; Widodo, W.S.; Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Armansyah, A.; Girsang, E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019, 6, 111–117. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Statistical Database; FAO: Rome, Italy, 2019. [Google Scholar]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Deb, L.; Tripathi, A.; Bhowmik, D.; Dutta, A.S.; Sampath, K.K.P. No titleanti-inflammatory activity of n-butanol fraction of Prunus persica L. aqueous extract. Pharm. Res. 2010, 4, 74–78. [Google Scholar]
- Bendaikha, S.; Gadaut, M.; Harakat, D.; Magid, A. Acylated flavonol glycosides from the flower of Elaeagnus angustifolia L. Phytochemistry 2014, 103, 129–136. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Baek, H.-S.; Rho, H.-S.; Yoo, J.-W.; Ahn, S.-M.; Lee, J.-Y.; Lee, J.-A.; Kim, M.-K.; Kim, D.-H.; Chang, I.-S. The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. Bull. Korean Chem. Soc. 2008, 29, 43–46. [Google Scholar]
- Iván, G.; Szabadka, Z.; Ördög, R.; Grolmusz, V.; Naray-Szabo, G. Four spatial points that define enzyme families. Biochem. Biophys. Res. Commun. 2009, 383, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farasat, A.; Ghorbani, M.; Gheibi, N.; Shariatifar, H. In silico assessment of the inhibitory effect of four flavonoids (Chrysin, Naringin, Quercetin, Kaempferol) on tyrosinase activity using the MD simulation approach. BioTechnologia 2020, 101, 193–204. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical assessment of new topical cream containing two essential oils combined with tretinoin in the treatment of acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Ingredients | F1 | F2 |
|---|---|---|
| Span 80 | 1.5% | 1.5% |
| Cetearath-20 | 3.5% | 3.5% |
| Liq.Paraffin | 7% | 7% |
| Beeswax | 2% | 2% |
| Cetostearyl alcohol | 6% | 6% |
| Biocrol ws2 | 1% | 1% |
| SLN | 2% | 5% |
| Propylene glycol | 3% | 3% |
| Purified water | 74% | 71% |
| Group | Treatment |
|---|---|
| G1 | Normal; saline treatment, not irradiated with UV |
| G2 | Negative control; saline treatment, irradiated with UV |
| G3 | Positive control; treated with a commercial cream (La Roche-Posay TM Active Vitamin C 10% Dermatological Anti-wrinkle Concentrate Intensive), irradiated with UV |
| G4 | Treated with 2% PPEE-SLNs cream (low dose), irradiated with UV |
| G5 | Treated with 5% PPEE-SLNs cream (high dose), irradiated with UV |
| Anti-Elastase | Anti-Collagenase | Anti-Tyrosinase | ||||
|---|---|---|---|---|---|---|
| % Inhibition | IC50 | % Inhibition | IC50 | % Inhibition | IC50 | |
| PPEE | 86.12 ± 1.42 | 55.88 ± 2.16 | 80.61 ± 1.28 | 311.91 ± 3.19 | 65.03 ± 1.85 | 362.5 ± 1.95 |
| PPEE-SLNs | 89.02 ± 2.31 | 54.01 ± 2.16 | 87.03 ± 1.31 | 283.92 ± 4.33 | 78.12 ± 1.50 | 303.03 ± 4.15 |
| KDPAG | 89.15 ± 1.26 | 49.80 ± 2.9 | 91.12 ± 2.45 | 267.66 ± 3.5 | 85.74 ± 2.10 | 290.19 ± 5.09 |
| Respective positive control | 92.52 ± 4.63 | 44.92 ± 1.71 | 79.82 ± 2.63 | 315.12 ± 2.21 | 76.52 ± 0.83% | 321.65 ± 3.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. https://doi.org/10.3390/antiox10030436
Mostafa ES, Maher A, Mostafa DA, Gad SS, Nawwar MAM, Swilam N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants. 2021; 10(3):436. https://doi.org/10.3390/antiox10030436
Chicago/Turabian StyleMostafa, Eman S., Ahmed Maher, Dalia A. Mostafa, Sameh S. Gad, Mahmoud A.M. Nawwar, and Noha Swilam. 2021. "A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare" Antioxidants 10, no. 3: 436. https://doi.org/10.3390/antiox10030436
APA StyleMostafa, E. S., Maher, A., Mostafa, D. A., Gad, S. S., Nawwar, M. A. M., & Swilam, N. (2021). A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants, 10(3), 436. https://doi.org/10.3390/antiox10030436