Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RCJ and GCJ
2.3. Determination of Total Phenolics
2.4. Determination of Ascorbic Acid
2.5. Determination of Glucosinolates
2.6. Determination of Total Anthocyanin
2.7. Extraction and HPLC-MS Analysis of Anthocyanins
2.8. Cell Culture and Viability Assay
2.9. RCJ and GCJ Pre-Treatment and H2O2-Induced Oxidative Injury Model
2.10. Assay of LDH in H2O2-Induced Caco-2 Cells
2.11. Strains and Growth Conditions
2.12. Lifespan Assay
2.13. Stress Resistance Assay
2.14. Autofluorescence
2.15. Quantification of Intracellular ROS
2.16. Body Length and Brood Size Assays
2.17. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.18. Data Analysis
3. Results
3.1. Total Phenolics, Ascorbic Acid, Glucosinolates, and Anthocyanins Contents in RCJ and GCJ and Identification of Anthocyanins in RCJ
3.2. Identification of Anthocyanins in RCJ
3.3. RCJ exhibited Antioxidant Activity in Caco-2 Cells
3.4. RCJ enhanced the Oxidative and Thermal Stress Resistance
3.5. RCJ and GCJ Attenuated Autofluorescence Accumulation but Do Not Affected ROS Level
3.6. RCJ and GCJ Decreased Brood Size and Increased Body Length
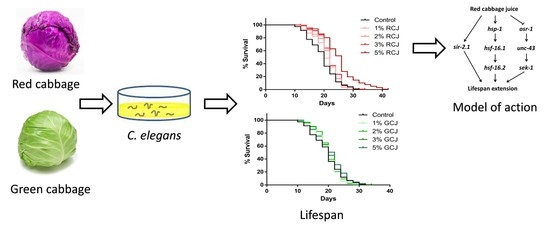
3.7. RCJ Extend the Lifespan of Wild-Type C. elegans
3.8. Genetic Requirements for Increased Survival from RCJ Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.-J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.). Lwt Food Sci. Technol. 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Al-Dosari, M.S. Red cabbage (Brassica oleracea L.) mediates redox-sensitive amelioration of dyslipidemia and hepatic injury induced by exogenous cholesterol administration. Am. J. Chin. Med. 2014, 42, 189–206. [Google Scholar] [CrossRef] [Green Version]
- Cruz, A.B.; Pitz, H.d.S.; Veber, B.; Bini, L.A.; Maraschin, M.; Zeni, A.L.B. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm. Biol. 2016, 54, 3033–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankhari, J.M.; Thounaojam, M.C.; Jadeja, R.N.; Devkar, R.V.; Ramachandran, A. Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J. Sci. Food Agric. 2012, 92, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, N.; Zhang, Z.; Jing, P. Effects of dietary cyanidin-3-diglucoside-5-glucoside complexes with rutin/Mg(II) against H2O2-induced cellular oxidative stress. Food Res. Int. 2019, 126, 108591. [Google Scholar] [CrossRef]
- Qian, B.J.; Wu, C.F.; Lu, M.M.; Xu, W.; Jing, P. Effect of complexes of cyanidin-3-diglucoside-5-glucoside with rutin and metal ions on their antioxidant activities. Food Chem. 2017, 232, 545–551. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, X.; Xiao, J.; Zhong, Q.; Kuang, Y.; Cao, Y.; Chen, Y. Effects on longevity extension and mechanism of action of carnosic acid in Caenorhabditis elegans. Food Funct. 2019, 10, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Kim, S.K.; Berdichevsky, A.; Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 2005, 9, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, J.; Xiang, Y.; Xiang, L.; Liu, Y.; He, X.; Zhou, X.; Liu, X.; Huang, Z. Extracts of Tsai Tai (Brassica chinensis): Enhanced antioxidant activity and anti-aging effects both in vitro and in Caenorhabditis elegans. Food Funct. 2016, 7, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; He, Z.; He, S.; Jing, P. Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties. Food Res. Int. 2019, 116, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Denardin, C.C.; Hirsch, G.E.; da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Ruan, S.-Y.; Dong, Y.; Zhang, X.-G.; Yue, J.; Kan, J.-Q.; Slavin, M.; Yu, L. Optimization of purification conditions of radish (Raphanus sativus L.) anthocyanin-rich extracts using chitosan. Lwt Food Sci. Technol. 2011, 44, 2097–2103. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yang, X.; Xu, W.; Cai, X.; Wang, M.; Xu, Y.; Yu, P.; Zhang, J.; Zheng, Y.; Chen, J.; et al. Evaluation of the effects of three sulfa sweeteners on the lifespan and intestinal fat deposition in C. elegans. Food Res. Int. 2019, 122, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.G.; Huang, J.A.; Li, J.; Yu, P.H.; Xiong, Z.; Zhang, J.W.; Gong, Y.S.; Liu, Z.H.; Chen, J.H. Black tea increased survival of Caenorhabditis elegans under stress. J. Agric. Food Chem. 2014, 62, 11163–11169. [Google Scholar] [CrossRef] [PubMed]
- Vayndorf, E.M.; Lee, S.S.; Liu, R.H. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Upadhyay, A.K.; Bahadur, A.; Singh, B.; Singh, K.P.; Rai, M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci. Hortic. 2006, 108, 233–237. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Jang, M.; Kim, K.H.; Kim, G.H. Antioxidant Capacity of Thistle (Cirsium japonicum) in Various Drying Methods and their Protection Effect on Neuronal PC12 Cells and Caenorhabditis elegans. Antioxidants 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Caland, R.B.; Cadavid, C.O.M.; Carmona, L.; Pena, L.; de Paula Oliveira, R. Pasteurized orange juice rich in carotenoids protects Caenorhabditis elegans against oxidative stress and beta-amyloid toxicity through direct and indirect mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 5046280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.B.L.; Gruber, J.; Tang, S.Y.; Schaffer, S.; Ong, R.L.S.; Fong, S.; Ng, L.F.; Cheah, I.; Halliwell, B. Ageing in nematodes: Do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology 2010, 11, 17–30. [Google Scholar] [CrossRef]
- Guha, S.; Natarajan, O.; Murbach, C.G.; Dinh, J.; Wilson, E.C.; Cao, M.; Zou, S.; Dong, Y. Supplement timing of cranberry extract plays a key role in promoting Caenorhabditis elegans healthspan. Nutrients 2014, 6, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging 2016, 8, 889. [Google Scholar] [CrossRef] [Green Version]
- Labuschagne, C.F.; Brenkman, A.B. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res. Rev. 2013, 12, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016, 78, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Pietsch, K.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Diversity of Polyphenol Action in Caenorhabditis elegans: Between Toxicity and Longevity. J. Nat. Prod. 2011, 74, 1713–1720. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Rose, M.R. Evolution of senescence: Late survival sacrificed for reproduction. Philos. Trans. R. Soc. Lond. Ser. BBiol. Sci. 1991, 332, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, Y.; Azat, R.; Zheng, X. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxidative Med. Cell. Longev. 2017, 2017, 7956158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, H.; Roxo, M.; Krstin, S.; Rohrig, T.; Richling, E.; Wink, M. An anthocyanin-rich extract of Acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Ewald, C.Y.; Castillo-Quan, J.I.; Blackwell, T.K. Untangling longevity, dauer, and healthspan in Caenorhabditis elegans insulin/IGF-1-signalling. Gerontology 2018, 64, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gami, M.S.; Wolkow, C.A. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell 2006, 5, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L.C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tissenbaum, H.A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006, 127, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Choi, E.; Lee, D.; Jeong, D.E.; Jang, S.K.; Lee, S.J. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell 2013, 12, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Aballay, A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc. Nat. Acad. Sci. USA 2006, 103, 13092–13097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, A.; Bandhakavi, S.; Jabbar, S.; Shah, R.; Beitel, G.J.; Morimoto, R.I. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004, 167, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Cao, M.; Kane, R.M.; Savino, A.M.; Zou, S.; Dong, Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age 2013, 35, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Gruber, J.; Ng, L.F.; Poovathingal, S.K.; Halliwell, B. Deceptively simple but simply deceptive–Caenorhabditis elegans lifespan studies: Considerations for aging and antioxidant effects. Febs Lett. 2009, 583, 3377–3387. [Google Scholar] [CrossRef] [Green Version]
- Saul, N.; Möller, S.; Cirulli, F.; Berry, A.; Luyten, W.; Fuellen, G. Health and longevity studies in C. elegans: The “healthy worm database” reveals strengths, weaknesses and gaps of test compound-based studies. Biogerontology 2021, 22, 215–236. [Google Scholar] [CrossRef] [PubMed]







| RCJ | GCJ | |
|---|---|---|
| Phenolics a | 29.95 ± 0.94 | 17.20 ± 0.15 * |
| Ascorbic acid | 30.55 ± 0.03 | 21.68 ± 0.17 * |
| Glucosinolates b | 70.21 ± 3.64 | 59.66 ± 1.04 * |
| Anthocyanins c | 33.87 ± 0.60 | none |
| Peak a | Retention Time (min) | [M]+ (m/z) | Fragments (m/z) | Tentative Identification |
|---|---|---|---|---|
| 1 | 4.49 | 773.21 | 287.05 | Cyanidin 3-diglucoside-5-glucoside |
| 2 | 6.60 | 1081.3 | 919.25/502.86/449.11/287.05 | Cyanidin 3-(caffeoyl)(p-coumaroyl)-diglucoside-5-glucoside |
| 3 | 6.77 | 1111.31 | 1111.31/949.26/606.18/449.11/287.05 | Cyanidin 3-(caffeoyl)(feruloyl)-diglucoside-5-glucoside |
| 4 | 8.13 | 919.25 | 919.25/757/287.05 | Cyanidin 3-(p-coumaroyl)-diglucoside-5-glucoside |
| 5 | 8.31 | 949.26 | 949.26/787.2/449.11/287.05 | Cyanidin 3-(feruloyl)-diglucoside-5-glucoside |
| 6 | 8.36 | 979.27 | 979.27/817.22/449.11/287.05 | Cyanidin 3-(sinapoyl)-diglucoside-5-glucoside |
| 7 | 9.15 | 1185.306 | 1185.33/1023.28/993.27/569.31/449.11/287.05 | Cyanidin 3-(sinapoyl)-diglucoside-5-(sinapoyl)-glucoside |
| Condition | Treatment | Number | Mean Lifespan (Days) a | % of Control |
|---|---|---|---|---|
| N2 | 0 | 116 | 19.57 ± 0.45 | |
| 1% RCJ | 150 | 20.63 ± 0.35 | 5.40 | |
| 2% RCJ | 159 | 21.60 ± 0.32 * | 10.37 | |
| 3% RCJ | 148 | 22.12 ± 0.29 * | 13.04 | |
| 5% RCJ | 157 | 25.08 ± 0.48 * | 28.18 | |
| 1% GCJ | 196 | 19.60 ± 0.45 | 0.16 | |
| 2% GCJ | 208 | 20.09 ± 0.26 | 2.65 | |
| 3% GCJ | 227 | 20.26 ± 0.26 | 3.51 | |
| 5% GCJ | 155 | 20.65 ± 0.48 | 5.50 | |
| GR1370 | 0 | 102 | 15.98 ± 0.27 | |
| 5% RCJ | 105 | 17.41 ± 0.11 * | 8.95 | |
| VC199 | 0 | 88 | 18.82 ± 0.40 | |
| 5% RCJ | 70 | 18.88 ± 0.72 | 0.32 | |
| MT2605 | 0 | 77 | 20.47 ± 0.62 | |
| 5% RCJ | 76 | 21.50 ± 0.24 | 5.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Jiao, S.; Jing, P. Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans. Antioxidants 2021, 10, 930. https://doi.org/10.3390/antiox10060930
Zhang N, Jiao S, Jing P. Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans. Antioxidants. 2021; 10(6):930. https://doi.org/10.3390/antiox10060930
Chicago/Turabian StyleZhang, Nan, Shunshan Jiao, and Pu Jing. 2021. "Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans" Antioxidants 10, no. 6: 930. https://doi.org/10.3390/antiox10060930
APA StyleZhang, N., Jiao, S., & Jing, P. (2021). Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans. Antioxidants, 10(6), 930. https://doi.org/10.3390/antiox10060930