Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings
Abstract
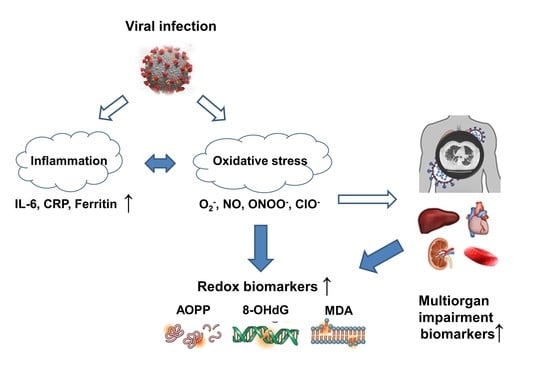
:1. Introduction
2. Materials and Methods
3. Results
3.1. Time Course of Redox Biomarkers in Patients with COVID-19 Pneumonia
3.2. The Correlation of Redox Biomarkers with Inflammatory Biomarkers and Multiorgan Impairment Biomarkers in Patients with COVID-19 Pneumonia at Different Time Points
3.3. The Association of Redox Biomarkers with CT Pulmonary Patterns in COVID-19 Pneumonia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and Immune Response: Focus on Oxidative Stress, Apoptosis and Autophagy. Phytother. Res. 2020, 34, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Bhattacharyya, S. Impact of Thiol–Disulfide Balance on the Binding of COVID-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor. ACS Omega 2020, 5, 16292–16298. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 Tissue Atlases Reveal SARS-CoV-2 Pathology and Cellular Targets. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C Levels in Patients with SARS-CoV-2-Associated Acute Respiratory Distress Syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy–Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Kalem, A.K.; Kayaaslan, B.; Neselioglu, S.; Eser, F.; Hasanoglu, İ.; Aypak, A.; Akinci, E.; Akca, H.N.; Erel, O.; Guner, R. A Useful and Sensitive Marker in the Prediction of COVID-19 and Disease Severity: Thiol. Free Radic. Biol. Med. 2021, 166, 11–17. [Google Scholar] [CrossRef]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef]
- Giustarini, D.; Santucci, A.; Bartolini, D.; Galli, F.; Rossi, R. The Age-Dependent Decline of the Extracellular Thiol-Disulfide Balance and Its Role in SARS-CoV-2 Infection. Redox Biol. 2021, 41, 101902. [Google Scholar] [CrossRef]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Umar Bindawa, K.; Ahmed, A. Deficiency of Antioxidants and Increased Oxidative Stress in COVID-19 Patients: A Cross-Sectional Comparative Study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 205031212199124. [Google Scholar] [CrossRef]
- Shang, W.; Dong, J.; Ren, Y.; Tian, M.; Li, W.; Hu, J.; Li, Y. The Value of Clinical Parameters in Predicting the Severity of COVID-19. J. Med. Virol. 2020, 92, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Sies, H.; Ferrucci, L.; Benzing, T. COVID-19 Mortality as a Fingerprint of Biological Age. Ageing Res. Rev. 2021, 67, 101308. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, A.C.; Lipinski, A.L.; Vasconcellos, F.T.F.; Marqueze, L.F.; Cunha, E.B.B.; Campos, A.C.; Oliveira, C.F.; Amaral, A.N.M.; Baena, C.P.; Telles, J.P.; et al. Susceptibility of the Patients Infected with SARS-CoV2 to Oxidative Stress and Possible Interplay with Severity of the Disease. Free Radic. Biol. Med. 2021, 165, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Oliva, A.; Cangemi, R.; Ceccarelli, G.; Pignatelli, P.; Carnevale, R.; Cammisotto, V.; Lichtner, M.; Alessandri, F.; De Angelis, M.; et al. Nox2 Activation in COVID-19. Redox Biol. 2020, 36, 101655. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020; p. 10. [Google Scholar]
- Miljanovic, D.; Milicevic, O.; Loncar, A.; Abazovic, D.; Despot, D.; Banko, A. The First Molecular Characterization of Serbian SARS-CoV-2 Isolates from a Unique Early Second Wave in Europe. Front. Microbiol. 2021, 12, 1526. [Google Scholar] [CrossRef]
- Shao, H.; Lan, D.; Duan, Z.; Liu, Z.; Min, J.; Zhang, L.; Huang, J.; Su, J.; Chen, S.; Xu, A. Upregulation of Mitochondrial Gene Expression in PBMC from Convalescent SARS Patients. J. Clin. Immunol. 2006, 26, 546–554. [Google Scholar] [CrossRef]
- Smith, J.T.; Willey, N.J.; Hancock, J.T. Low Dose Ionizing Radiation Produces Too Few Reactive Oxygen Species to Directly Affect Antioxidant Concentrations in Cells. Biol. Lett. 2012, 8, 594–597. [Google Scholar] [CrossRef] [Green Version]
- Borges do Nascimento, I.J.; Cacic, N.; Abdulazeem, H.M.; von Groote, T.C.; Jayarajah, U.; Weerasekara, I.; Esfahani, M.A.; Civile, V.T.; Marusic, A.; Jeroncic, A.; et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J. Clin. Med. 2020, 9, 941. [Google Scholar] [CrossRef] [Green Version]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Vlahos, R.; Selemidis, S. NADPH Oxidases as Novel Pharmacologic Targets against Influenza A Virus Infection. Mol. Pharmacol. 2014, 86, 747–759. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.-H.; Martinez, S.S.; Parsons, M.; Jayaweera, D.T.; Campa, A.; Baum, M.K. Relationship of Oxidative Stress with HIV Disease Progression in HIV/HCV Co-Infected and HIV Mono-Infected Adults in Miami. Int. J. Biosci. Biochem. Bioinforma. 2012, 2, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Dysangco, A.; Liu, Z.; Stein, J.H.; Dubé, M.P.; Gupta, S.K. HIV Infection, Antiretroviral Therapy, and Measures of Endothelial Function, Inflammation, Metabolism, and Oxidative Stress. PLoS ONE 2017, 12, e0183511. [Google Scholar] [CrossRef]
- Liu, M.; Chen, F.; Liu, T.; Chen, F.; Liu, S.; Yang, J. The Role of Oxidative Stress in Influenza Virus Infection. Microbes Infect. 2017, 19, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Checconi, P.; Salzano, S.; Bowler, L.; Mullen, L.; Mengozzi, M.; Hanschmann, E.-M.; Lillig, C.H.; Sgarbanti, R.; Panella, S.; Nencioni, L.; et al. Redox Proteomics of the Inflammatory Secretome Identifies a Common Set of Redoxins and Other Glutathionylated Proteins Released in Inflammation, Influenza Virus Infection and Oxidative Stress. PLoS ONE 2015, 10, e0127086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza Virus Replication in Lung Epithelial Cells Depends on Redox-sensitive Pathways Activated by NOX4-derived ROS. Cell. Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Metzger, D.W. Influenza Infection Suppresses NADPH Oxidase–Dependent Phagocytic Bacterial Clearance and Enhances Susceptibility to Secondary Methicillin-Resistant Staphylococcus Aureus Infection. J. Immunol. 2014, 192, 3301–3307. [Google Scholar] [CrossRef] [Green Version]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the Use of N-acetylcysteine in Both Prevention and Adjuvant Therapy of COVID-19. FASEB J. 2020, 34, 13185–13193. [Google Scholar] [CrossRef] [PubMed]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Majewski, W.; Strzyżewski, K.W.; Kasprzak, M.P.; Iskra, M. Advanced Oxidation Protein Products and Carbonylated Proteins as Biomarkers of Oxidative Stress in Selected Atherosclerosis-Mediated Diseases. Biomed. Res. Int. 2017, 2017, 4975264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Goud, P.T.; Bai, D.; Abu-Soud, H.M. A Multiple-Hit Hypothesis Involving Reactive Oxygen Species and Myeloperoxidase Explains Clinical Deterioration and Fatality in COVID-19. Int. J. Biol. Sci. 2021, 17, 62–72. [Google Scholar] [CrossRef]
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.-M.; Bahreini, E. A Comprehensive Review of COVID-19 Characteristics. Biol. Proced. Online 2020, 22, 19. [Google Scholar] [CrossRef]
- Campbell, C.M.; Kahwash, R. Will Complement Inhibition Be the New Target in Treating COVID-19–Related Systemic Thrombosis? Circulation 2020, 141, 1739–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]




| Parameter | |
|---|---|
| Gender | |
| Male, n (%) | 24 (41) |
| Female, n (%) | 34 (59) |
| Age a | 48.20 ± 13.46 |
| Systemic Arterial Hypertension, n (%) | 8 (14) |
| BMI (kg/m2) a | 28.03 ± 5.34 |
| Smoking, n (%) b | 1 (2) |
| Hospitalization | 42 (72) |
| Therapy | On Admission | 7 Days Upon Admission | 14 Days Upon Admission |
|---|---|---|---|
| Oxygenation, n (%) | 17 (29) | 14 (24) | 1 (2) |
| Vasoactive drugs, n (%) | 8 (14) | 8 (14) | 8 (14) |
| Hydroxychloroquine n (%) | 10 (17) | - | - |
| Low weight heparin, n (%) | 40 (69) | 40 (68) | 17 (29) |
| Aspirin, n (%) | 16 (28) | 17 (29) | 30 (52) |
| Tocilizumab, n (%) | - | 1 (2) | - |
| Favipiravir, n (%) | 15 (26) | - | - |
| Supplementation (Vitamin D, Vitamin C and Zn), n (%) | 58 (100) | 58 (100) | 58 (100) |
| Laboratory Parameters | On Admission | 7 Days Upon Admission | 14 Days Upon Admission | p Value |
|---|---|---|---|---|
| Inflammatory Biomarkers | ||||
| WBC (n) | 5.0 ± 1.8 | 6.1 ± 1.9 | 6.6 ± 2.0 | <0.001 |
| Neutrophils (n) | 3.0 ± 1.7 | 3.6 ± 1.8 | 3.8 ± 1.7 | 0.005 |
| Lymphocytes (n) | 1.4 ± 0.4 | 1.8 ± 0.5 | 1.4 ± 0.4 | <0.001 |
| Monocytes (n) | 0.4 ± 0.2 | 0.5 ± 0.8 | 0.6 ± 0.2 | <0.001 |
| NLR | 2.1 (0.4–8.9) | 1.8 (0.8–24.6) | 1.9 (0.9–8.5) | >0.05 |
| IL-6 (pg/mL) | 7.6 (1–112) | 4 (1–45) | 3 (1–41) | <0.001 |
| CRP (mg/L) | 7.2 (0.1–79.8) | 3.9 (0.3–83.2) | 1.8 (0.02–38.7) | <0.001 |
| Ferritin (ng/mL) | ||||
| Female | 80.5 (13.0–1500.9) | 105.0 (13.0–612.0) | 76.5 (12.0–536.0) | 0.011 |
| Male | 350.0 (136.0–1500.9) | 557.0 (156.0–1500.9) | 401.0 (103.0–1500.9) | 0.004 |
| Multiorgan impairment biomarkers | ||||
| Urea (mmol/L) | 4.5 ± 1.7 | 4.5 ± 2.3 | 5.3 ± 2.7 | 0.002 |
| Creatinine (µmol/L) | 85.1 ± 21.2 | 78.8 ± 16.0 | 83.8 ± 16.8 | 0.001 |
| ALT (U/L) | 33 (17–165) | 59 (19–518) | 53 (17–253) | 0.002 |
| AST (U/L) | 22.5 (11–95) | 35.5 (11–310) | 26 (10–141) | 0.018 |
| LDH (U/L) | 189.5 (99–473) | 192.5 (86–581) | 174 (91–411) | 0.001 |
| CK (U/L) | 66.5 (12–565) | 54.5 (15–809) | 53.5 (19–518) | <0.001 |
| Redox biomarkers | ||||
| MDA (ng/mL) | 709.8 (178.4–1542.1) | 477.5 (140.64–1583.6) | 547.5 (230.6–1570.8) | 0.002 |
| 8-OHdG (ng/mL) | 7.7 (4.6–16.9) | 8.9 (3.4–16.9) | 8.6 (4.6–16.7) | 0.006 |
| AOPP (µM) | 265.9 (117.3–779.67) | 239.8 (31.3–623.8) | 265.05 (108.2–830.3) | <0.001 |
| On Admission | MDA (ng/mL) Rho | 8-OHdG (ng/mL) Rho | AOPP (µM) Rho |
|---|---|---|---|
| IL 6 (pg/mL) | −0.08 | −0.04 | 0.07 |
| CRP (mg/L) | −0.13 | −0.19 | 0.17 |
| Ferritin (ng/mL) | −0.23 | 0.24 | 0.05 |
| Lymphocytes (n) | 0.18 | 0.03 | −0.10 |
| Neutrophils (n) | −0.06 | 0.08 | −0.08 |
| Monocytes (n) | 0.08 | −0.03 | 0.07 |
| 7 days upon admission | |||
| IL 6 (pg/mL) | −0.10 | −0.19 | 0.18 |
| CRP (mg/L) | −0.18 | −0.02 | 0.32 * |
| Ferritin (ng/mL) | −0.09 | 0.06 | 0.32 * |
| Lymphocytes (n) | 0.22 | 0.16 | −0.22 |
| Neutrophils (n) | −0.06 | −0.01 | 0.27 * |
| Monocytes (n) | 0.23 | 0.18 | 0.04 |
| 14 days upon admission | |||
| IL 6 (pg/mL) | 0.02 | 0.19 | 0.28 * |
| CRP (mg/L) | −0.07 | −0.01 | 0.21 |
| Ferritin (ng/mL) | −0.19 | 0.01 | 0.26 * |
| Lymphocytes (n) | 0.09 | 0.07 | 0.21 |
| Neutrophils (n) | −0.21 | 0.01 | 0.18 |
| Monocytes (n) | 0.16 | 0.25 | 0.28 * |
| On Admission | MDA (ng/mL) Rho | 8-OHdG (ng/mL) Rho | AOPP (µM) Rho |
|---|---|---|---|
| Urea (mmol/L) | −0.16 | 0.18 | 0.01 |
| Creatinine (µmol/L) | −0.22 | 0.39 * | 0.13 |
| ALT (U/L) | −0.01 | 0.30 * | 0.01 |
| AST (U/L) | −0.05 | 0.23 | 0.09 |
| LDH (U/L) | −0.10 | 0.01 | 0.01 |
| CK (U/L) | −0.36 | −0.01 | 0.17 |
| 7 days upon admission | |||
| Urea (mmol/L) | −0.06 | 0.06 | −0.03 |
| Creatinine (µmol/L) | −0.09 | 0.02 | 0.15 |
| ALT (U/L) | 0.09 | 0.08 | 0.09 |
| AST (U/L) | 0.03 | 0.01 | 0.01 |
| LDH (U/L) | −0.21 | −0.19 | 0.01 |
| CK (U/L) | −0.19 | −0.05 | −0.14 |
| 14 days upon admission | |||
| Urea (mmol/L) | −0.18 | 0.05 | 0.01 |
| Creatinine (µmol/L) | −0.09 | 0.20 | 0.25 |
| ALT (U/L) | 0.01 | 0.01 | 0.23 |
| AST (U/L) | −0.02 | −0.16 | 0.44 * |
| LDH (U/L) | −0.01 | −0.15 | 0.29 * |
| CK (U/L) | −0.17 | 0.03 | −0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosanovic, T.; Sagic, D.; Djukic, V.; Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Bukumiric, Z.; Lalosevic, M.; Djordjevic, M.; Coric, V.; Simic, T. Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings. Antioxidants 2021, 10, 1126. https://doi.org/10.3390/antiox10071126
Kosanovic T, Sagic D, Djukic V, Pljesa-Ercegovac M, Savic-Radojevic A, Bukumiric Z, Lalosevic M, Djordjevic M, Coric V, Simic T. Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings. Antioxidants. 2021; 10(7):1126. https://doi.org/10.3390/antiox10071126
Chicago/Turabian StyleKosanovic, Tijana, Dragan Sagic, Vladimir Djukic, Marija Pljesa-Ercegovac, Ana Savic-Radojevic, Zoran Bukumiric, Miodrag Lalosevic, Marjana Djordjevic, Vesna Coric, and Tatjana Simic. 2021. "Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings" Antioxidants 10, no. 7: 1126. https://doi.org/10.3390/antiox10071126
APA StyleKosanovic, T., Sagic, D., Djukic, V., Pljesa-Ercegovac, M., Savic-Radojevic, A., Bukumiric, Z., Lalosevic, M., Djordjevic, M., Coric, V., & Simic, T. (2021). Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings. Antioxidants, 10(7), 1126. https://doi.org/10.3390/antiox10071126