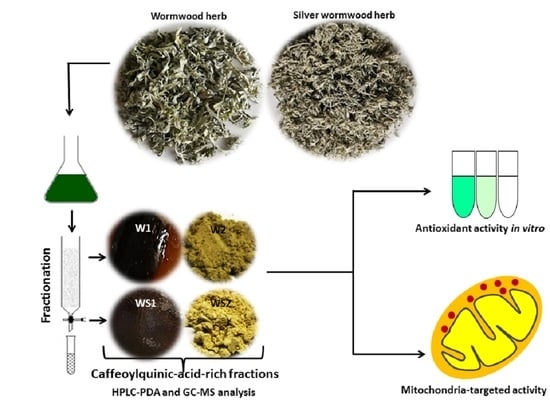
Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Plant Material
2.3. Production of Dry Extracts
2.4. Fractionation of Caffeoylquinic Acids
2.5. HPLC-PDA Analysis for Characterization of Caffeoylquinic Acids and GC–MS Analysis for Essential Oil Components
2.6. Antioxidant Activity of Caffeoylquinic-Acid-Rich Fractions and Acetone Extracts
2.7. Preparation of Isolated Kidney Mitochondria
2.8. Measurement of Mitochondrial Respiration
2.9. Measurement of Cytochrome c Reduction Level
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of Caffeoylquinic-Acid-Rich Fractions and Acetone Extracts
3.2. Antioxidant Activity of Fractions and Acetone Extracts
3.3. Effects of Fractions on Kidney Mitochondrial Respiration Rates
3.4. Measurement of the Cytochrome c Reduction Level of Caffeoylquinic-Acid Rich Fractions and Acetone Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Compound | Calibration Curve | Coefficient of Determination (R2) | Rt (min) | λ max * (nm) |
|---|---|---|---|---|
| Chlorogenic acid | y = 47,700x + 2650 | 0.99992 | 14.41 | 245 sh, 296 sh, 325 |
| Neochlorogenic acid | y = 25,600x + 1730 | 0.99944 | 10.52 | 245 sh, 296 sh, 325 |
| 4-O-caffeoylquinic acid | y = 60,200x − 1620 | 0.99966 | 14.95 | 245 sh, 296 sh, 325 |
| 4,5-Dicaffeoylquinic acid | y = 40,400x − 9600 | 0.99997 | 30.98 | 245 sh, 296 sh, 325 |
| 3,4-Dicaffeoylquinic acid | Y = 56,700x − 6790 | 0.99999 | 35.35 | 245 sh, 296 sh, 327 |
| 3,5-Dicaffeoylquinic acid | y = 79,100x − 3690 | 0.99989 | 34.02 | 245 sh, 296 sh, 327 |
| Luteolin-7-glucoside | y = 54,300x + 1040 | 0.99998 | 26.92 | 254 sh, 267 sh, 348 |
| Luteolin-7-rutinoside | y = 40,300x − 3980 | 0.99999 | 23.80 | 254 sh, 267 sh, 348 |

References
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.; Rao, R. Antiviral and Immunomodulation Effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef] [PubMed]
- Hrytsyk, R.A.; Kutsyk, R.V.; Yurchyshyn, O.I.; Struk, O.A.; Kireev, I.V.; Grytsyk, A.R. The investigation of antimicrobial and antifungal activity of some Artemisia L. species. Pharmacia 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Sainz, P.; Andrés, M.F.; Díaz, M.; Bailén, M.; Rocha, J.N.; Martínez-Díaz, R.A.; González-Coloma, A. Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef] [Green Version]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y. Chondroprotective and anti-nociceptive effects of caffeoylquinic acid in osteoarthritis by downregulating catabolic activity and oxidative damage in chondrocytes. Biomed. Pharmacother. 2017, 93, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure-Activity Relationship of Caffeoylquinic Acids on the Accelerating Activity on ATP Production. Chem. Pharm. Bull. 2011, 59, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; Dilisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Tsubata, T. Involvement of Reactive Oxygen Species (ROS) in BCR Signaling as a Second Messenger. B Cells Immun. Toler. 2020, 1254, 37–46. [Google Scholar] [CrossRef]
- Andrés Juan, C.; Manuel Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular Sciences The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, anging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Kumar, N.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxidants Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.C.S.; Deus, C.M.; Borges, F.; Oliveira, P. Mitochondria: Targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic-based antioxidants. Int. J. Biochem. Cell Biol. 2018, 97, 98–103. [Google Scholar] [CrossRef]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxidative Med. Cell. Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Bou-Teen, D.; Kaludercic, N.; Weissman, D.; Turan, B.; Maack, C.; Di Lisa, F.; Ruiz-Meana, M. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free. Radic. Biol. Med. 2021, 167, 109–124. [Google Scholar] [CrossRef]
- Škėmienė, K.; Jablonskienė, G.; Liobikas, J.; Borutaitė, V. Protecting the Heart Against Ischemia/Reperfusion-Induced Necrosis and Apoptosis: The Effect of Anthocyanins. Medicina 2013, 49, 15–18. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Park, J.-Y.; Kim, D.; Kang, M.; Seong, H.-A.; Seo, K.; Ji, Y.-J. Neuroprotective Effects of Coreopsis lanceolata Flower Extract against Oxidative Stress-Induced Apoptosis in Neuronal Cells and Mice. Antioxidants 2021, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef] [Green Version]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Caffeic Acid Phenethyl Ester Protects Kidney Mitochondria against Ischemia/Reperfusion Induced Injury in an In Vivo Rat Model. Antioxidants 2021, 10, 747. [Google Scholar] [CrossRef]
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uckun, F.M.; Saund, S.; Windlass, H.; Trieu, V. Repurposing Anti-Malaria Phytomedicine Artemisinin as a COVID-19 Drug. Front. Pharmacol. 2021, 12, 649532. [Google Scholar] [CrossRef]
- Krishna, S.; Augustin, Y.; Wang, J.; Xu, C.; Staines, H.M.; Platteeuw, H.; Kamarulzaman, A.; Sall, A.; Kremsner, P. Repurposing Antimalarials to Tackle the COVID-19 Pandemic. Trends Parasitol. 2020, 37, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Uzun, T.; Toptaş, O.; Türkoğlu, A. Could Artesunate Have a Positive Effect on the Neurological Complications Related to Infection When It Is Used in the Treatment of COVID-19? ACS Chem. Neurosci. 2020, 11, 4001–4006. [Google Scholar] [CrossRef]
- Magaña, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against Gram-Positive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B. 5-Caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J. Nutr. Biochem. 2009, 20, 800–805. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.-H.; Lee, K. Characterization of Caffeoylquinic Acids from Lepisorus thunbergianus and Their Melanogenesis Inhibitory Activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Toyama, D.O.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Gaeta, H.H.; Toyama, M.H. Effect of Chlorogenic Acid (5-Caffeoylquinic Acid) Isolated fromBaccharis oxyodontaon the Structure and Pharmacological Activities of Secretory Phospholipase A2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, 2014, 726585. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-Z.; Zhao, D.-X.; Xiang, J.; Zhang, M.; Zhang, C.-F.; Xu, X.-H. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated fromTussilago farfara. Pharm. Biol. 2015, 54, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, S.; Castilho, P. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of Caffeoylquinic Acids in Coffee Brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Bai, J.-P.; Zhang, Q.; Hu, X.-L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.-H.; Zhao, Q.-C. Caffeoylquinic acid derivatives from the roots of Arctium lappa L. (burdock) and their structure–activity relationships (SARs) of free radical scavenging activities. Phytochem. Lett. 2016, 15, 159–163. [Google Scholar] [CrossRef]
- Tamayose, C.I.; Dos Santos, E.A.; Roque, N.; Costa-Lotufo, L.V.; Ferreira, M.J.P. Caffeoylquinic Acids: Separation Method, Antiradical Properties and Cytotoxicity. Chem. Biodivers. 2019, 16, e1900093. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasileva, A.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic Acids and Flavonoids of Fringed Sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS Profile, HPLC-DAD Quantification, in Vitro Digestion Stability, and Antioxidant Capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Joo, T.; Jhoo, J.-W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and Cytoprotective Effects of the Di-O-Caffeoylquinic Acid Family: The Mechanism, Structure–Activity Relationship, and Conformational Effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant profiles of leaves and inflorescences of native, invasive and hybrid Solidago species. Ind. Crop. Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Yamasoba, T. Mitochondria-Targeted Antioxidants for Treatment of Hearing Loss: A Systematic Review. Antioxidants 2019, 8, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Torres, A.; Noriega, L.G.; Delgadillo-Puga, C.; Tovar, A.R.; Navarro-Ocaña, A. Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes. Molecules 2021, 26, 319. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; E Puig, P.; Didelot, C.M.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 877–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagoa, R.; Gutierrez-Merino, C. Cytochrome c Reducing Agents and Antiapoptotic Action of Antioxidants. In Cytochrome c: Roles and Therapeutic Implications; Nova Science Publishers: Hauppauge, NY, USA, 2019. [Google Scholar]
- Zazeri, G.; Povinelli, A.P.R.; Pavan, N.M.; de Carvalho, D.R.; Cardoso, C.L.; Ximenes, V.F. Experimental studies and computational modeling on cytochrome c reduction by quercetin: The role of oxidability and binding affinity. J. Mol. Struct. 2021, 1244, 130995. [Google Scholar] [CrossRef]
- Banik, S.; Rahman, M.; Sikder, T.; Saito, T.; Kurasaki, M. Protective effects of ajwain (Trachyspermum ammi L.) extract against cadmium-induced cytotoxicity and apoptosis in PC12 cells. J. Herb. Med. 2021, 26, 100423. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, H.; Yao, W.; Gao, X. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Cell Biol. Int. 2007, 32, 304–311. [Google Scholar] [CrossRef]








| Compound | Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.008 µg/mL (ng/mL) | 0.8 µg/mL (ng/mL) | |||||||
| Caffeoylquinic Acid-Rich Fractions | ||||||||
| W1 | WS1 | W2 | WS2 | W1 | WS1 | W2 | WS2 | |
| Chlorogenic acid | 0.52 ± 0.024 | 1.13 ± 0.038 | ND | ND | 51.86 ± 2.362 | 112.52 ± 3.842 | ND | ND |
| Neochlorogenic acid | 0.03 ± 0.001 | 0.05 ± 0.002 | ND | ND | 2.99 ± 0.136 | 5.24 ± 0.179 | ND | ND |
| 4-O-caffeoylquinic acid | 0.02 ± 0.001 | 0.06 ± 0.002 | ND | ND | 1.69 ± 0.077 | 6.11 ± 0.209 | ND | ND |
| 4,5-Dicaffeoylquinic acid | ND | ND | 0.14 ± 0.009 | 0.82 ± 0.008 | ND | ND | 13.81 ± 0.898 | 81.70 ± 0.804 |
| 3,4-Dicaffeoylquinic acid | ND | ND | 1.00 ± 0.010 | 1.43 ± 0.006 | ND | ND | 100.24 ± 0.967 | 143.02 ± 0.643 |
| 3,5-Dicaffeoylquinic acid | ND | ND | 1.53 ± 0.012 | 3.08 ± 0.007 | ND | ND | 152.69 ± 1.177 | 307.58 ± 0.727 |
| Compound | Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 32.5 µg/mL (ng/mL) | 65 µg/mL (ng/mL) | |||||||
| Caffeoylquinic Acid-Rich Fractions | ||||||||
| W1 | WS1 | W2 | WS2 | W1 | WS1 | W2 | WS2 | |
| Chlorogenic acid | 2074.56 ± 95.95 | 4500.41 ± 156.07 | ND | ND | 4149.11 ± 191.90 | 9000.81 ± 312.14 | ND | ND |
| Neochlorogenic acid | 119.53 ± 5.53 | 209.71 ± 7.27 | ND | ND | 239.05 ± 11.06 | 419.41 ± 14.54 | ND | ND |
| 4-O-caffeoylquinic acid | 67.71 ± 3.13 | 244.48 ± 8.48 | ND | ND | 135.41 ± 6.26 | 488.96 ± 16.96 | ND | ND |
| 4,5-Dicaffeoylquinic acid | ND | ND | 552.42 ± 36.49 | 3268.09 ± 32.67 | ND | ND | 1104.83 ± 72.98 | 6536.18 ± 65.34 |
| 3,4-Dicaffeoylquinic acid | ND | ND | 4009.70 ± 39.28 | 5720.63 ± 26.11 | ND | ND | 8019.39 ± 78.56 | 11,441.25 ± 52.22 |
| 3,5-Dicaffeoylquinic acid | ND | ND | 6107.69 ± 47.82 | 12,303.19 ± 29.52 | ND | ND | 12,215.38 ± 95.65 | 24,606.37 ± 59.05 |
| Assay(μM TE/g DW) | W1 | WS1 | W2 | WS2 |
|---|---|---|---|---|
| ABTS | 367 ± 58 #&@ | 745 ± 83 *@ | 679 ± 134 *@ | 1693 ± 59 *#& |
| FRAP | 5385 ± 168 &@ | 5505 ± 175 &@ | 6952 ± 162 *#@ | 6052 ± 81 *#& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. https://doi.org/10.3390/antiox10091405
Kamarauskaite J, Baniene R, Raudone L, Vilkickyte G, Vainoriene R, Motiekaityte V, Trumbeckaite S. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants. 2021; 10(9):1405. https://doi.org/10.3390/antiox10091405
Chicago/Turabian StyleKamarauskaite, Justina, Rasa Baniene, Lina Raudone, Gabriele Vilkickyte, Rimanta Vainoriene, Vida Motiekaityte, and Sonata Trumbeckaite. 2021. "Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.)" Antioxidants 10, no. 9: 1405. https://doi.org/10.3390/antiox10091405
APA StyleKamarauskaite, J., Baniene, R., Raudone, L., Vilkickyte, G., Vainoriene, R., Motiekaityte, V., & Trumbeckaite, S. (2021). Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants, 10(9), 1405. https://doi.org/10.3390/antiox10091405