Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma
Abstract
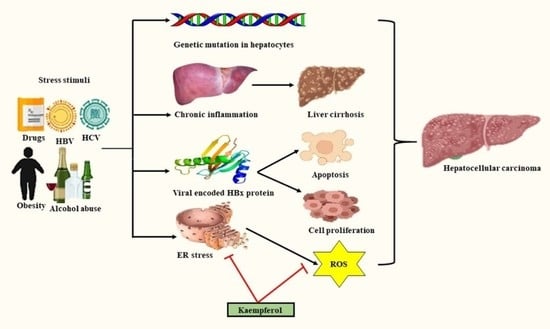
:1. Introduction
2. Regulated Cell Death and Kaempferol
3. Oxidative Stress (OS) in Hepatocarcinogenesis
3.1. HBV and HCV Related HCC and Oxidative Stress
3.2. Non-Alcoholic Steatohepatitis (NASH) Related HCC and OS
4. Antioxidant Potential of Kaempferol in Preventing HCC
4.1. Peroxisome Proliferator-Activated Receptor (PPAR)
4.2. Nuclear Factor Erythroid Related Factor 2 (Nrf2)
5. Role of Oxidative Stress in Endoplasmic Reticulum (ER) Hemostasis
6. Endoplasmic Reticulum Stress Signaling Pathways
6.1. IRE1α-XBP1 Pathway
6.2. PERK-eIF2α-ATF4 Pathway
6.3. ATF6 Pathway
7. Role of Kaempferol in ER Stress and Oxidative Stress-Induced Apoptosis
8. Modulation of ER Stress and Autophagy Machinery by Kaempferol
9. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Moratalla-López, N.; Lorenzo, C.; Alonso, G.L.; Sánchez, A.M. Kaempferol glycosides in Crocus: Sources, biosynthesis, and uses. In Kaempferol: Biosynthesis, Food Sources and Therapeutic Uses, 2016th ed.; Nova Science Publisher: Hauppauge, NY, USA, 2016; pp. 151–195. [Google Scholar]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Sumaiya, S.; Sharma, A.; Naved, T.; Sarwat, M. Amelioration of liver ailments by saffron (Crocus sativus) and its secondary metabolites. In Saffron: The Age Old Panacea in New Light; Sarwat, M., Sumaiya, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic β-cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective effects of kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of glycogen synthase kinase-3. Oxidative Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Liu, Y.; Sun, A.; Du, Y.; Ye, M.; Pu, X.; Qi, X. Intestinal absorption and neuroprotective effects of kaempferol-3-O-rutinoside. RSC Adv. 2017, 7, 31408–31416. [Google Scholar] [CrossRef] [Green Version]
- Chandramohan, G.; Al-Numair, K.S.; Alsaif, M.A.; Veeramani, C. Antidiabetic effect of kaempferol a flavonoid compound, on streptozotocin-induced diabetic rats with special reference to glycoprotein components. Prog. Nutr. 2015, 17, 50–57. [Google Scholar]
- Vellosa, J.C.; Regasini, L.O.; Khalil, N.M.; Bolzani, V.D.; Khalil, O.A.; Manente, F.A.; Netto, H.P.; Oliveira, O.M. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quim. 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Tatsimo, S.J.; de Dieu Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Choi, K.C. Anti-cancer effect and underlying mechanism (s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013, 29, 229–234. [Google Scholar] [CrossRef]
- Patel, S.; Sarwat, M.; Khan, T.H. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): The molecular perspective. Crit. Rev. Oncol./Hematol. 2017, 115, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G980–G988. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, X.; Li, H.; Yan, Y.; Hong, X.; Lin, Z. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int. J. Immunopathol. Pharmacol. 2018, 32. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; González, C.A.; Bamia, C.; Trepo, E.; Nöthlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L.; et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and smoking-related cancer risk: A meta-analysis. PLoS ONE 2013, 8, e75604. [Google Scholar] [CrossRef] [Green Version]
- Nair, B.; Anto, R.J.; Sabitha, M.; Nath, L.R. Kaempferol-mediated sensitization enhances chemotherapeutic efficacy of sorafenib against hepatocellular carcinoma: An in silico and in vitro approach. Adv. Pharm. Bull. 2020, 10, 472–476. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Ciccarone, F.; Castelli, S.; Ciriolo, M.R. Oxidative stress-driven autophagy acROSs onset and therapeutic outcome in hepatocellular carcinoma. Oxidative Med. Cell. Longev. 2019, 2019, 6050123. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta -Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Shah, A.M.; Channon, K.M. Free radicals and redox signalling in cardiovascular disease. Heart 2004, 90, 486–487. [Google Scholar] [CrossRef] [Green Version]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, 209–219. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The Neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 2020, 442, 1–13. [Google Scholar] [CrossRef]
- Bowler, R.P.; Crapo, J.D. Oxidative stress in allergic respiratory diseases. J. Allergy Clin. Immunol. 2002, 110, 349–356. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Chandan, K.; Sarwat, M. Role of miRNA and long non-coding RNA in hepatocellular carcinoma. Curr. Pharm. Des. 2020, 26, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of host and pathogen-derived micrornas in immune regulation during infectious and inflammatory diseases. Front. Immunol. 2020, 10, 3081–3095. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Serenari, M.; Cucchetti, A.; Ercolani, G. Treatment options for recurrence of hepatocellular carcinoma after surgical resection: Review of the literature and current recommendations for management. Hepatoma Res. 2020, 6, 26. [Google Scholar] [CrossRef]
- Zheng, S.; Xie, Q.; Cheng, J. Salvage liver transplant for hepatocellular carcinoma: Rescues and benefits. Transl. Gastroenterol. Hepatol. 2018, 3, 65. [Google Scholar] [CrossRef]
- Paul, S.B.; Manjunatha, Y.C.; Acharya, S.K. Palliative treatment in advanced hepatocellular carcinoma: Has it made any difference? Trop. Gastroenterol. 2010, 30, 125–134. [Google Scholar]
- Gupta, M.; Akhtar, J.; Sarwat, M. MicroRNAs: Regulators of immunological reactions in hepatocellular carcinoma. Semin. Cell Dev. Biol. 2021. [Google Scholar] [CrossRef]
- Sasaki, Y. Does oxidative stress participate in the development of hepatocellular carcinoma? J. Gastroenterol. 2006, 41, 1135–1148. [Google Scholar] [CrossRef]
- Napoletano, F.; Baron, O.; Vandenabeele, P.; Mollereau, B.; Fanto, M. Intersections between regulated cell death and autophagy. Trends Cell Biol. 2019, 29, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, L.; Liu, K. Tumor-suppressing effects of autophagy on hepatocellular carcinoma. Liver Res. 2018, 2, 157–160. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Li, X.; Li, B.; Li, Y.; Zhang, X.; Ma, Y.; Peng, X.; Jin, H.; Li, H. New insights into autophagy in hepatocellular carcinoma: Mechanisms and therapeutic strategies. Am. J. Cancer Res. 2019, 9, 1329–1353. [Google Scholar] [PubMed]
- Guo, H.; Lin, W.; Zhang, X.; Zhang, X.; Hu, Z.; Li, L.; Duan, Z.; Zhang, J.; Ren, F. Kaempferol induces hepatocellular carcinoma cell death via endoplasmic reticulum stress-CHOP-autophagy signaling pathway. Oncotarget 2017, 8, 82207–82216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, H.O.; Huang, H.; Tsung, A. Autophagy: Dual response in the development of hepatocellular carcinoma. Cells 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquardt, J.U.; Edlich, F. Predisposition to apoptosis in hepatocellular carcinoma: From mechanistic insights to therapeutic strategies. Front. Oncol. 2019, 13, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Yamamoto, K. Control of oxidative stress in hepatocellular carcinoma: Helpful or harmful? World J. Hepatol. 2015, 7, 968–979. [Google Scholar] [CrossRef]
- Gentric, G.; Maillet, V.; Paradis, V.; Couton, D.; L’hermitte, A.; Panasyuk, G.; Fromenty, B.; Celton-Morizur, S.; Desdouets, C. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Tarocchi, M.; Galli, A. Oxidative stress as a mechanism for hepatocellular carcinoma. In Liver Pathophysiology; Elsevier B.V: Amsterdam, The Netherlands, 2017; pp. 279–287. [Google Scholar]
- Marra, M.; Sordelli, I.M.; Lombardi, A.; Lamberti, M.; Tarantino, L.; Giudice, A.; Stiuso, P.; Abbruzzese, A.; Sperlongano, R.; Accardo, M.; et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: An overview. J. Transl. Med. 2011, 9, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative stress and liver cancer: Etiology and therapeutic targets. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.L.; Yu, D.Y. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J. Gastroenterol. 2010, 16, 4932–4937. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, S.; Duan, X.; Yang, C.; Xu, M.; Chen, L. Macrophage Phenotypes and Hepatitis B Virus Infection. J. Clin. Transl. Hepatol. 2020, 8, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, W.; Wang, Y.; Qiao, L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014, 345, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.K.; Zheng, H.; Li, S.; Chen, R.; Liu, X.; Li, Y.; Lee, N.P.; Lee, W.H.; Ariyaratne, P.N.; Tennakoon, C.; et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 2012, 44, 765–769. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, Y.J. C-terminal region of HBx is crucial for mitochondrial DNA damage. Cancer Lett. 2013, 331, 76–83. [Google Scholar] [CrossRef]
- Shen, S.; Niso-Santano, M.; Adjemian, S.; Takehara, T.; Malik, S.A.; Minoux, H.; Souquere, S.; Mariño, G.; Lachkar, S.; Senovilla, L.; et al. Cytoplasmic STAT3 represses autophagy by inhibiting PKR activity. Mol. Cell 2012, 48, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Formanowicz, D.; Radom, M.; Rybarczyk, A.; Formanowicz, P. The role of Fenton reaction in Ros-induced toxicity underlying atherosclerosis-modeled and analyzed using a Petri net-based approach. Biosystems 2018, 165, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Negro, F. Natural history of NASH and HCC. Liver Int. 2020, 40, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.S.; Jeong, J.Y.; Kim, M.J.; Lee, S.M.; Nam, W.H.; Myung, S.J.; Kim, J.G.; Yang, S.K.; Kim, J.H.; Suh, D.J. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int. J. Cancer 2010, 127, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, J.; Liu, L.; Li, Q.; Rong, W.; Wang, L.; Wang, Y.; Zang, M.; Wu, Z.; Zhang, Y.; et al. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS ONE 2012, 7, e50035. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.S.D.; Cirino, J.P.G.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 2143–2158. [Google Scholar]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar]
- Zang, Y.; Zhang, D.; Yu, C.; Jin, C.; Igarashi, K. Antioxidant and hepatoprotective activity of kaempferol 3-O-β-D-(2, 6-di-O-α-L-rhamnopyranosyl) galactopyronoside against carbon tetrachloride-induced liver injury in mice. Food Sci. Biotechnol. 2017, 26, 1071–1076. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xuan, Y.H.; Luo, M.X.; Ni, X.G.; Ling, L.Q.; Hu, S.J.; Chen, J.Q.; Xu, J.Y.; Jiang, L.Y.; Si, W.Z.; et al. Kaempferol alleviates acute alcoholic liver injury in mice by regulating intestinal tight junction proteins and butyrate receptors and transporters. Toxicology 2020, 429, 152338. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Hu, M.; Xu, Y.; Zhao, S.; Sun, Y.; Wang, B.; Hu, J.; Li, Y. Drug interaction study of flavonoids toward OATP1B1 and their 3D structure activity relationship analysis for predicting hepatoprotective effects. Toxicology 2020, 437, 152445. [Google Scholar] [CrossRef]
- Singab, A.N.; Youssef, D.T.; Noaman, E.; Kotb, S. Hepatoprotective effect of flavonol glycosides rich fraction from egyptianVicia calcarata desf. Against CCI 4-induced liver damage in rats. Arch. Pharmacal Res. 2005, 28, 791–798. [Google Scholar] [CrossRef]
- Cho, S.S.; Yang, J.H.; Seo, K.H.; Shin, S.M.; Park, E.Y.; Cho, S.S.; Jo, G.U.; Eo, J.H.; Park, J.S.; Oh, D.S.; et al. Cudrania Tricuspidata Extract and Its Major Constituents Inhibit Oxidative Stress-Induced Liver Injury. J. Med. Food 2019, 22, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.F.; Bian, Y.Q.; Wu, R.; Sun, Y.; Chen, X.L.; Yang, M.D.; Zhang, Q.R.; Hu, Y.; Sun, M.Y.; Su, S.B. Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomed. Pharmacother. 2019, 114, 108863. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol attenuates liver fibrosis by inhibiting activin receptor–like kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Qiu, H.; Zhu, L.; Ren, Z.; Zhao, W.; Zhang, T.; Liu, L. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food Funct. 2015, 6, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I.; Lakka, A.; Tsakalof, A.; Simos, G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem. Biophys. Res. Commun. 2010, 398, 74–78. [Google Scholar] [CrossRef]
- Yusof, H.M.; Ng, M.S.; Lam, T.W.; Kassim, M.N. Hypolipidemic effects of quercetin and kaempferol in human hepatocellular carcinoma (HepG2) cells. Int. Food Res. J. 2018, 25, 241–245. [Google Scholar]
- Wonganan, O.; He, Y.J.; Shen, X.F.; Wongkrajang, K.; Suksamrarn, A.; Zhang, G.L.; Wang, F. 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol. Appl. Pharmacol. 2017, 336, 31–39. [Google Scholar] [CrossRef]
- Du, Y.C.; Lai, L.; Zhang, H.; Zhong, F.R.; Cheng, H.L.; Qian, B.L.; Tan, P.; Xia, X.M.; Fu, W.G. Kaempferol from Penthorum chinense Pursh suppresses HMGB1/TLR4/NF-κB signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef]
- Shrivastava, S.; Uthra, C.; Reshi, M.; Shukla, S. Protective Role of Kaempferol against Acrylamide Intoxication. Free Radic. Antioxid. 2017, 7, 36–42. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, M.; Xiang, H.; Zheng, P.; Wu, T.; Ji, G. Integrative transcriptomics and metabolomics explore the mechanism of kaempferol on improving nonalcoholic steatohepatitis. Food Funct. 2020, 2020, 10058–10069. [Google Scholar] [CrossRef]
- Wei, T.; Xiong, F.F.; Wang, S.D.; Wang, K.; Zhang, Y.Y.; Zhang, Q.H. Flavonoid ingredients of Ginkgo biloba leaf extract regulate lipid metabolism through Sp1-mediated carnitine palmitoyltranferase 1A up-regulation. J. Biomed. Sci. 2014, 21, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [Green Version]
- Mello, T.; Materozzi, M.; Galli, A. PPARs and mitochondrial metabolism: From NAFLD to HCC. PPAR Res. 2016, 2016, 7403230. [Google Scholar] [CrossRef]
- Xiao, Y.B.; Cai, S.H.; Liu, L.L.; Yang, X.; Yun, J.P. Decreased expression of peroxisome proliferator-activated receptor alpha indicates unfavorable outcomes in hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Tzeng, T.F.; Liou, S.S.; Chang, Y.S.; Liu, I.M. bIntroduction. Planta Med. 2011, 77, 1876–1882. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Yang, J.H.; Ki, S.H. Role of the Nrf2-ARE pathway in liver diseases. Oxidative Med. Cell. Longev. 2013, 2013, 763257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, E.; Karim, M.R.; Teeli, A.S.; Śmiech, M.; Leszczynski, P.; Winiarczyk, D.; Parvanov, E.D.; Atanasov, A.G.; Taniguchi, H. Molecular Mechanisms Underlying Hepatocellular Carcinoma Induction by Aberrant NRF2 Activation-Mediated Transcription Networks: Interaction of NRF2-KEAP1 Controls the Fate of Hepatocarcinogenesis. Int. J. Mol. Sci. 2020, 21, 5378. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, M.; Tuteja, N. Calnexin: A versatile calcium binding integral membrane chaperone of endoplasmic reticulum. Calcium Bind. Proteins 2007, 2, 36–50. [Google Scholar]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K. The unfolded protein response: The dawn of a new field. Proc. Japan Acad. Ser. B 2015, 91, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Sarwat, M.; Naqvi, A.R. Heterologous Expression of Rice Calnexin (OsCNX) Confers Drought Tolerance in Nicotiana tabacum. Mol. Biol. Rep. 2013, 40, 5451–5464. [Google Scholar] [CrossRef]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: A focus on the protective roles of heme oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Creighton, T.E.; Hillson, D.A.; Freedman, R.B. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J. Mol. Biol. 1980, 142, 43–62. [Google Scholar] [CrossRef]
- Hwang, C.J.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef]
- Bhandary, B.; Marahatta, A.; Kim, H.R.; Chae, H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2013, 14, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.G.; Travers, K.J.; Weissman, J.S. Ero1p: A novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1998, 1, 171–182. [Google Scholar] [CrossRef]
- Plaisance, V.; Brajkovic, S.; Tenenbaum, M.; Favre, D.; Ezanno, H.; Bonnefond, A.; Bonner, C.; Gmyr, V.; Kerr-Conte, J.; Gauthier, B.R.; et al. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL. PLoS ONE 2016, 11, e0163046. [Google Scholar] [CrossRef] [Green Version]
- Moslehi, A.; Komeili-movahed, T.; Moslehi, M. Antioxidant effects of amygdalin on tunicamycin-induced endoplasmic reticulum stress in the mice liver: Cross talk between endoplasmic reticulum stress and oxidative stress. J. Rep. Pharm. Sci. 2019, 8, 298–302. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Zou, Y.; Guo, H.; Zhang, B.; Xia, C.; Zhang, H.; Yang, W.; Xu, C. NFκB/Orai1 facilitates endoplasmic reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Front. Cell Dev. Biol. 2019, 7, 202–215. [Google Scholar] [CrossRef]
- Riaz, T.A.; Junjappa, R.P.; Handigund, M.; Ferdous, J.; Kim, H.R.; Chae, H.J. Role of endoplasmic reticulum stress sensor ire1α in cellular physiology, calcium, ROS signaling, and metaflammation. Cells 2020, 9, 1160. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Wang, X.Z.; Wang, T.; Chen, J.J.; Xie, X.Y.; Hu, H.; Yu, F.; Liu, H.L.; Jiang, X.Y.; Fan, H.D. Molecular signal networks and regulating mechanisms of the unfolded protein response. J. Zhejiang Univ.-Sci. B 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.Y.; Hsu, Y.W.; Liao, C.L.; Lin, Y.L. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J. Virol. 2006, 80, 11868–11880. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Nenseth, H.Z.; Qu, S.; Kuzu, O.F.; Frahnow, T.; Simon, L.; Greene, S.; Zeng, Q.; Fazli, L.; Rennie, P.S.; et al. IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun. 2019, 10, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, G.M.; Brodsky, J.L. The evolving role of ubiquitin modification in endoplasmic reticulum-associated degradation. Biochem. J. 2017, 474, 445–469. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Chen, Z.; Li, L.; Liu, H.; Zhang, R.; Cheng, Q.; Xu, D.; Wu, L. Unravel the molecular mechanism of XBP1 in regulating the biology of cancer cells. J. Cancer 2019, 10, 2035–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, A.M.; Healy, S.J.; Jäger, R.; Samali, A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012, 134, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Marelli, M.M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Green, R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019, 3, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.; Gradzik, K.; Kabzinski, J.; Przybylowska-Sygut, K.; Majsterek, I. The role of the ER-induced UPR pathway and the efficacy of its inhibitors and inducers in the inhibition of tumor progression. Oxidative Med. Cell. Longev. 2019, 2019, 5729710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, I.; Weiwad, M.; Prell, E.; Ferrari, D.M. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6–CHOP pathway of stress response. Apoptosis 2014, 19, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Guo, H.; Ren, F.; Zhang, L.; Zhang, X.; Yang, R.; Xie, B.; Li, Z.; Hu, Z.; Duan, Z.; Zhang, J. Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mol. Med. Rep. 2016, 13, 2791–2800. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef]
- Niering, P.; Michels, G.; Wätjen, W.; Ohler, S.; Steffan, B.; Chovolou, Y.; Kampkötter, A.; Proksch, P.; Kahl, R. Protective and detrimental effects of kaempferol in rat H4IIE cells: Implication of oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2005, 209, 114–1122. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, H.H.; Chyau, C.C.; Wang, Z.H.; Chen, J.H. Aqueous Extract of Pepino Leaves Ameliorates Palmitic Acid-Induced Hepatocellular Lipotoxicity via Inhibition of Endoplasmic Reticulum Stress and Apoptosis. Antioxidants 2021, 10, 903. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12–28. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L. Endoplasmic reticulum stress and autophagy. Autophagy Biol. Dis. 2019, 1206, 167–177. [Google Scholar]
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Pop, L.; Raduly, L.; Barbat, A.; Moldovan, A.; et al. Inhibitory effect of CAPE and kaempferol in colon cancer cell lines—Possible implications in new therapeutic strategies. Int. J. Mol. Sci. 2019, 20, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res. 2020, 34, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Wang, X.J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget. 2017, 8, 86227–86239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, H.; Shao, M.; Lu, Y.; Wang, J.; Wu, T.; Ji, G. Kaempferol Alleviates Steatosis and Inflammation during Early Non-Alcoholic Steatohepatitis Associated With Liver X Receptor α-Lysophosphatidylcholine Acyltransferase 3 Signaling Pathway. Front. Pharmacol. 2021, 59, 146–156. [Google Scholar]




| Cause | Factors Activated | Mechanism Involved | Impact on Oxidative Stress and HCC | References |
|---|---|---|---|---|
| Hepatitis B Virus (HBV) | HBx protein | ↑ Oncogene expression, activation of macrophages to release proinflammatory cytokines (IL-1β, IL-6, CXCL-8, and TNF-α), activation of apoptosis | ↑ ROS and HCC | [61] |
| Gene mutation | Induce ER stress | |||
| Hepatitis C Virus (HCV) | Core Protein | Activates signaling pathways (TNFR, PKR, and STAT3 pathways), induces apoptosis, metastasis, and DNA damage. | ↑ ROS and HCC | [64,75] |
| Fe2+ accumulation | Fenton reaction (Iron toxicity) | |||
| NASH | Fatty toxicity | ↑ IL-17 | ↑ ROS and HCC | [71] |
| Central Obesity | Reduces the level of adiponectin, leading to increased cell growth, proliferation, and metastasis |
| Diseases Type | In Vitro/In Vivo Model | Mechanism of Action | Concentrations/Doses | References |
|---|---|---|---|---|
| Alcoholic liver injury | Mice | ↑ expression of butyrate receptors, transporters, and TJ proteins in the intestinal mucosa. | 25, 50 and 100 mg/kg | [83] |
| Alcoholic liver injury | ALI mice model | Increased antioxidant defense activity, decreased oxidative stress, and lipid peroxidation. | 10 and 20 mg/kg | [82] |
| Liver injury | Bosentan-induced rat liver injury model and HEK-293 cells | Inhibition of OATP1B1 transporter, maintaining a level of AST, ALT | 25 mg/kg and 1–150 μM | [84] |
| Liver injury | Male Swiss albino rats | Inhibition of lipid peroxidation caused by CCL4 reactive free radicals. | 25 mg/kg | [85] |
| Liver injury | Male ddY mice | ↓ TBARS and TNF-α level in CCL4 treated mice. | 4.9 mg/kg | [80] |
| Liver injury | Mice and HepG2 cells | Reduces AA+Fe-induced ROS production and reversed glutathione depletion, ↓ cell death. | 250 and 500 mg/kg and 100, 200 and 400 μM | [86] |
| Liver fibrosis | L02, LX2 and Rats | ↓ Protein levels of cleaved caspase-3, ↑ p-ERK1/2, PI3K, and Bcl-XL protein expression in TNF-α-stimulated L02 cells. The suppressed proliferation of LX2 cells and up-regulation of Bax and cleaved caspase-8. | 20 μM | [87] |
| Liver fibrosis | HSCs/Ccl4 induced mouse model | Down-regulation of hyaluronic acid, ALT, AST, Smad2/3. Inhibits collagen synthesis and activation of HSCs cells. Suppression of activin receptor-like kinase 5. | 2–10 μmol/L | [88] |
| Liver cancer | HepG2 | Apoptosis, reduced expression of miR-21, upregulation of PTEN expression and PI3K/AKT/mTOR signaling pathways inactivation. | 0, 25, 50, 75, and 100 μM | [20] |
| Liver cancer | HepG2 cells | ↑ PIG3 level at mRNA and protein level, ↑ROS production, cytochrome C release, ↓ mitochondrial membrane potential, upregulation of Bax/Bcl-2, activation of caspases-9 and -3, and maintaining the pro-oxidant activity. | 10, 20, 40 and 80 μM | [89] |
| Human hepatic cancer | SK-HEP-1 | ↑ protein levels of p-AMPK, LC3-II, Atg 5, Atg 7, Atg 12 and Beclin 1, ↓ level of CDK1, cyclin B, p-AKT, and p-Mtor. Downregulation of CDK1/Cyclin B pathways, Induces autophagy. | 0, 25, 50, 75 and 100 μM | [17] |
| HCC | Huh 7 | HIF-1a activity inactivation by cytoplasmic mislocalization and MAPK pathway inhibition. | 1–100 µM | [90] |
| HCC | HepG2 | ↑ The hypolipidemic effect through LDL-c uptake. | 15 µM | [91] |
| HCC | HepG2 cells | ↑ phosphorylation of JAK1, Tyk2, and STAT1/2, ↓ phosphorylation of STAT3, promoted endogenous IFN-α-regulated genes expression, ↓ expression of SOCS3, ↑the anti-proliferative effect of IFN-α, activation of the JAK/STAT signaling pathway | 10 µg/mL | [92] |
| Hepatotoxicity | Male C57BL/6 mice | Decreased level of ALT, AST. Induce hepatocellular damage, ↑ expression of antioxidant enzymes, and apoptosis. Reduces NLRP3 expression and pro-inflammatory factors. Inhibition of HMGBI/TLR4/NF-KB signaling pathway. | 30 and 60 mg/kg | [93] |
| Acrylamide hepatic intoxication | Wistar female albino rats | Reduced TBAR and GSH level | 5, 10, 20 and 40 mg/kg | [94] |
| Nonalcoholic steatohepatitis (NASH) | Male C57BL/6 mice | ↓ level of ALT, LDL, triglycerides, total cholesterol, lipid droplets and inflammatory cells infiltration in the liver, Upregulation of DEGs, Regulation of fatty acid degradation, expression of cytochrome P450, ↓ level of urinary proteins family (Mup17, Mup7, and Mup16). | 4 mg/mL | [95] |
| NAFLD | HepG2 cells | ↓ hepatic lipid accumulation, promote β oxidation in mitochondria and up-regulation of the expression of CPT1A | 20 μg/mL | [96] |
| Diseases Type | In Vitro/In Vivo Model | Mechanism of Action | Concentations/Doses | References |
|---|---|---|---|---|
| Acute liver failure | Murine ALF model induced by D-galactosamine/lipopolysaccharide mice | Regulation of ER stress-Grp78-CHOP pathway | 5 mg/kg | [132] |
| HCC | HepG2 | Apoptosis, and Upregulation of CHOP gene expression. | 0, 5, 10, 25 50 and 100 µM | [131] |
| HCC | H4IIE | H2O2 mediated lipid peroxidation leading to cell death and DNA damage, ↑ the activity of caspases-2, -3/7, -9, and -8/10, and apoptosis. | 5–25 µM | [133] |
| HCC | HepG2 and Huh 7 | ↑ The protein level of Atg5, Atg7, Beclin1, and Overexpression of CHOP induces autophagy. | 5~100 μM | [48] |
| NASH | HepG2 cells/C57BL/6 NASH mice model | Decresed expression of LXRα, LPCAT3 and ERS-related factors PERK, eIF2α, ATF6, ATF4, XBP1, CHOP, IRE1α and GRP78 and induction of apoptosis. | 20, 40, 60 μmol/L and 20 mg/kg | [141] |
| Hepatocellular lipotoxicity | HepG2 | Decreased ER stress, increased antioxidant ability and inhibited apoptosis. | 1, 5, 10, 100 µg/mL | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. https://doi.org/10.3390/antiox10091419
Sharma N, Biswas S, Al-Dayan N, Alhegaili AS, Sarwat M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants. 2021; 10(9):1419. https://doi.org/10.3390/antiox10091419
Chicago/Turabian StyleSharma, Nidhi, Subhrajit Biswas, Noura Al-Dayan, Alaa Saud Alhegaili, and Maryam Sarwat. 2021. "Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma" Antioxidants 10, no. 9: 1419. https://doi.org/10.3390/antiox10091419
APA StyleSharma, N., Biswas, S., Al-Dayan, N., Alhegaili, A. S., & Sarwat, M. (2021). Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants, 10(9), 1419. https://doi.org/10.3390/antiox10091419