Theoretical and Experimental Considerations for a Rapid and High Throughput Measurement of Catalase In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Modeling
2.2. Plant Material
2.3. Enzyme Activity Assay
2.4. Statistical Analysis
3. Results and Discussion
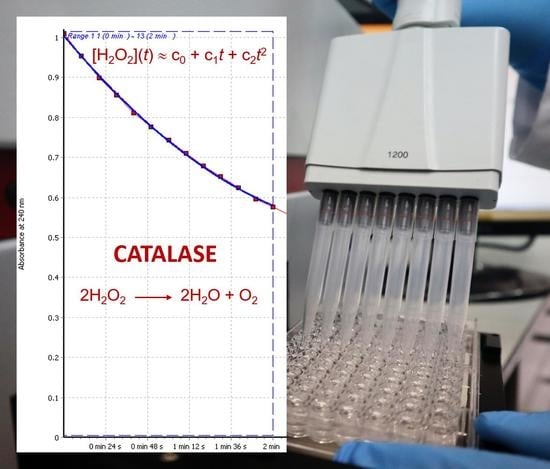
3.1. Theoretical Considerations for an Easy Fitting of the Experimental Data of the H2O2 Decomposition by Catalase with a Two-Degree Polynomial
3.2. Linear Dependence of the H2O2 Decomposition Rate on the Catalase Concentration
3.3. Experimental Considerations for a Rapid and High-Throughput Method to Measure the Catalase Activity in Microplate Readers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loew, O. A new enzyme of general occurrence in organisms. Science 1900, 11, 701–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Jennings, M.E.; Schaff, C.W.; Horne, A.J.; Lessner, F.H.; Lessner, D.J. Expression of a bacterial catalase in a strictly anaerobic methanogen significantly increases tolerance to hydrogen peroxide but not oxygen. Microbiology 2014, 160, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application—A review. Biocatal. Biotransform. 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. In Advances in Inorganic Chemistry; Sykes, A.G., Mauk, G., Eds.; Academic Press: San Diego, CA, USA, 2001; Volume 51, pp. 51–106. ISBN 0-12-023651-6. [Google Scholar]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally designed mimics of antioxidant manganoenzymes: Role of structural features in the quest for catalysts with catalase and superoxide dismutase activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 31055214. [Google Scholar] [CrossRef] [PubMed]
- Mireles, R.; Ramirez-Ramirez, J.; Alcalde, M.; Ayala, M. Ether oxidation by an evolved fungal heme-peroxygenase: Insights into substrate recognition and reactivity. J. Fungi 2021, 7, 608. [Google Scholar] [CrossRef] [PubMed]
- Feuers, R.J.; Pattillo, F.M.; Osborn, C.K.; Adams, K.L.; DeLuca, D.; Grady Smith, W. Application of an integrated rate equation to the inactivation of catalase. Free Radic. Biol. Med. 1993, 15, 223–226. [Google Scholar] [CrossRef]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Hohne, M.; Hendriks, J.H.M.; Palacios, N.; Cross, J.; Selbig, J.; Stitt, M. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathematica v.12.2; Wolfram Research: Champaign, IL, USA, 2021.
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Genotypic variability on grain yield and grain nutritional quality characteristics of wheat grown under elevated CO2 and high temperature. Plants 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Bolger, A.M.; Martínez-Carrasco, R.; Pérez, P.; Gutiérrez, E.; Usadel, B.; Morcuende, R. De novo transcriptome analysis of durum wheat flag leaves provides new insights into the regulatory response to elevated CO2 and high temperature. Front. Plant Sci. 2019, 10, e1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A.; Perez-Lopez, U.; Robredo, A.; Lacuesta, M.; et al. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant 2009, 135, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zor, T.; Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellado-Ortega, E.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Arellano, J.B. Solutions to decrease a systematic error related to AAPH addition in the fluorescence-based ORAC assay. Anal. Biochem. 2017, 519, 27–29. [Google Scholar] [CrossRef]
- R CoreTeam. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 16 November 2021).
- Bisswanger, H. Enzyme Kinetics: Principles and Methods; Wiley-VCH: Weinheim, Germany, 2009; Volume 46, ISBN 978-3-527-30343-4. [Google Scholar]
- Trawczyńska, I. New method of determining kinetic parameters for decomposition of hydrogen peroxide by catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Ghadermarzi, M.; Moosavi-Movahedi, A.A. Determination of the kinetic parameters for the “suicide substrate” inactivation of bovine liver catalase by hydrogen peroxide. J. Enzyme Inhib. Med. Chem. 1996, 10, 167–175. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, D.C.; Dennis, R.; Smith, W.G. Inactivation of an animal and a fungal catalase by hydrogen peroxide. Arch. Biochem. Biophys. 1995, 320, 129–134. [Google Scholar] [CrossRef]
- Miłek, J. Estimation of the kinetic parameters for H2O2 enzymatic decomposition and for catalase deactivation. Braz. J. Chem. Eng. 2018, 35, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Bénard, C.; Gibon, Y. Measurement of enzyme activities and optimization of continuous and discontinuous assays. Curr. Protoc. Plant Biol. 2016, 1, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef]
- Jammer, A.; Gasperl, A.; Luschin-Ebengreuth, N.; Heyneke, E.; Chu, H.; Cantero-Navarro, E.; Großkinsky, D.K.; Albacete, A.A.; Stabentheiner, E.; Franzaring, J.; et al. Simple and robust determination of the activity signature of key carbohydrate metabolism enzymes for physiological phenotyping in model and crop plants. J. Exp. Bot. 2015, 66, 5531–5542. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Riquelme, N.; Huerta-Retamal, N.; Gómez-Torres, M.J.; Martínez-Espinosa, R.M. Catalase as a molecular target for male infertility diagnosis and monitoring: An overview. Antioxidants 2020, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Donald Malevu, T.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A summary of new findings on the biological effects of selenium in selected animal species—A critical review. IJMS 2017, 18, 2209. [Google Scholar] [CrossRef]
- Salami, S.A.; Guinguina, A.; Agboola, J.O.; Omede, A.A.; Agbonlahor, E.M.; Tayyab, U. Review: In vivo and postmortem effects of feed antioxidants in livestock: A review of the implications on authorization of antioxidant feed additives. Animal 2016, 10, 1375–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Lane | n1 | n2 | Statistic | Df | p-Value | Significance |
|---|---|---|---|---|---|---|
| A | 30 | 30 | 1.26 | 29 | 0.2180 | ns |
| B | 30 | 30 | −0.12 | 29 | 0.9070 | ns |
| C | 30 | 30 | −1.02 | 29 | 0.3160 | ns |
| D | 30 | 30 | −5.18 | 29 | 0.0000 | *** |
| E | 30 | 30 | −3.70 | 29 | 0.0009 | *** |
| F | 30 | 30 | −3.16 | 29 | 0.0040 | ** |
| G | 30 | 30 | −4.10 | 29 | 0.0003 | *** |
| H | 30 | 30 | −4.76 | 29 | 0.0001 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendou, O.; Gutiérrez-Fernández, I.; Marcos-Barbero, E.L.; Bueno-Ramos, N.; González-Hernández, A.I.; Morcuende, R.; Arellano, J.B. Theoretical and Experimental Considerations for a Rapid and High Throughput Measurement of Catalase In Vitro. Antioxidants 2022, 11, 21. https://doi.org/10.3390/antiox11010021
Bendou O, Gutiérrez-Fernández I, Marcos-Barbero EL, Bueno-Ramos N, González-Hernández AI, Morcuende R, Arellano JB. Theoretical and Experimental Considerations for a Rapid and High Throughput Measurement of Catalase In Vitro. Antioxidants. 2022; 11(1):21. https://doi.org/10.3390/antiox11010021
Chicago/Turabian StyleBendou, Ouardia, Ismael Gutiérrez-Fernández, Emilio L. Marcos-Barbero, Nara Bueno-Ramos, Ana I. González-Hernández, Rosa Morcuende, and Juan B. Arellano. 2022. "Theoretical and Experimental Considerations for a Rapid and High Throughput Measurement of Catalase In Vitro" Antioxidants 11, no. 1: 21. https://doi.org/10.3390/antiox11010021
APA StyleBendou, O., Gutiérrez-Fernández, I., Marcos-Barbero, E. L., Bueno-Ramos, N., González-Hernández, A. I., Morcuende, R., & Arellano, J. B. (2022). Theoretical and Experimental Considerations for a Rapid and High Throughput Measurement of Catalase In Vitro. Antioxidants, 11(1), 21. https://doi.org/10.3390/antiox11010021