Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts
Abstract
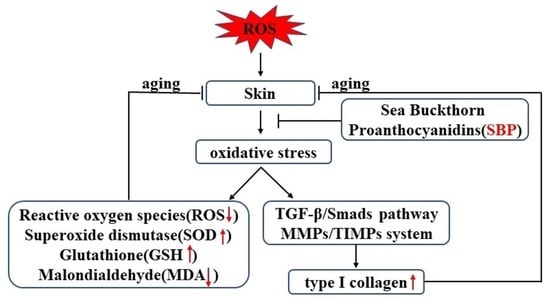
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cytotoxicity Assay of SBP
2.5. Establishment of Senescent Cell Models
2.6. Cell Treatment
2.7. Effects of SBP on H2O2-Induced Senescent HSFs
2.8. β-Galactosidase Staining Method to Verify the Effect of SBP on H2O2-Induced Senescent HSFs
2.9. ROS Assay
2.10. Determination of SOD Activity, GSH, and MDA Content
2.11. Col I Immunofluorescence Staining
2.12. Measurement of MMP-1, -3, and TIMP-1 Production
2.13. Western Blotting
2.14. Cell Wound Scratch Assay
2.15. Statistical Analysis
3. Results
3.1. Cytotoxicity Assay of SBP on HSFs and Establishment of Senescent Cell Models
3.2. SBP Favors the Restoration of Cell Morphology and Cell Viability in H2O2-Induced Senescent Cells
3.3. Effects of SBP on ROS, SOD Activity, GSH, and MDA in H2O2-Induced Senescent HSFs
3.4. SBP Up-Regulates the Expression of Col I in H2O2-Induced Aging HSFs and Inhibits Col I Degradation by Regulating the Expressions of MMP-1, -3, and TIMP-1
3.5. SBP Promotes the Synthesis of Type I Procollagen through the TGF-β1/Smads Pathway
3.6. SBP Enhances the Migration Ability of H2O2-Induced Senescent HSFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Fu, H.; Zhao, D.; Zhang, J.; Wang, C.; Wang, D.; Li, M. Protective Effects of Mogroside V on Oxidative Stress Induced by H2O2 in Skin Fibroblasts. Drug Des. Dev. Ther. 2021, 15, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Agarwal, A.; Uitto, J.; Mauviel, A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J. Biol. Chem. 1996, 271, 3272–3278. [Google Scholar] [CrossRef] [PubMed]
- Ignotz, R.A.; Massagué, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Sárdy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Floch, G.L.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2011, 131, 754–760. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Sheng, L.; Zhang, H.; Chen, F.; Zhu, J.; Ding, M. Lotus Seedpod Proanthocyanidins Protect Against Light-Induced Retinal Damage via Antioxidative Stress, Anti-Apoptosis, and Neuroprotective Effects. Med. Sci. Monit. 2021, 27, e935000. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, P.; Pi, S.; Guo, Y.; Pei, S.; Yang, W.; Chang, X.; Wang, L.; Chen, F. Proanthocyanidins Protect Against Cadmium-Induced Diabetic Nephropathy Through p38 MAPK and Keap1/Nrf2 Signaling Pathways. Front. Pharm. 2022, 12, 801048. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape seed proanthocyanidins reduced the overweight of C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar]
- Zhu, Y.L.; Yuen, M.; Li, W.; Yuen, H.; Wang, M.; Smith, D.; Peng, Q. Composition analysis and antioxidant activity evaluation of a high purity oligomeric procyanidin prepared from sea buckthorn by a green method. Curr. Res. Food Sci. 2021, 4, 840–851. [Google Scholar] [CrossRef]
- Childs, B.G.; Bussian, T.J.; Baker, D.J. Cellular Identification and Quantification of Senescence-Associated β-Galactosidase Activity In Vivo. Methods Mol. Biol. 2019, 1896, 31–38. [Google Scholar]
- Menicacci, B.; Laurenzana, A.; Chillà, A.; Margheri, F.; Peppicelli, S.; Tanganelli, E.; Fibbi, G.; Giovannelli, L.; Del Rosso, M.; Mocali, A. Chronic Resveratrol Treatment Inhibits MRC5 Fibroblast SASP-Related Protumoral Effects on Melanoma Cells. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1187–1195. [Google Scholar] [CrossRef]
- Pan, C.; Lang, H.; Zhang, T.; Wang, R.; Lin, X.; Shi, P.; Pang, X. Conditioned medium derived from human amniotic stem cells delays H2O2-induced premature senescence in human dermal fibroblasts. Int. J. Mol. Med. 2019, 44, 1629–1640. [Google Scholar] [CrossRef]
- Cui, Y.; Li, F.; Zhu, X.; Xu, J.; Muhammad, A.; Chen, Y.; Li, D.; Liu, B.; Wang, C.; Wang, Z.; et al. Alfalfa saponins inhibit oxidative stress-induced cell apoptosis through the MAPK signaling pathway. Redox Rep: Commun. Free Radic. Res. 2022, 27, 1–8. [Google Scholar] [CrossRef]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef]
- Blumberg, J. Use of biomarkers of oxidative stress in research studies. J. Nutr. 2004, 134, 3188S–3189S. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Spaulding, H.R. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020, 32, 101508. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Qi, J. Nano-Drug Design Based on the Physiological Properties of Glutathione. Molecules 2021, 26, 5567. [Google Scholar] [CrossRef]
- Alyethodi, R.R.; Sirohi, A.S.; Karthik, S.; Tyagi, S.; Perumal, P.; Singh, U.; Sharma, A.; Kundu, A. Role of seminal MDA, ROS, and antioxidants in cryopreservation and their kinetics under the influence of ejaculatory abstinence in bovine semen. Cryobiology 2021, 98, 187–193. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhao, X.Y.; Zhang, J.M.; Lu, C.; Feng, F.J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard Mater. 2022, 435, 129077. [Google Scholar] [CrossRef]
- Huang, X.; Ye, Y.; Zhang, J.; Zhang, X.; Ma, H.; Zhang, Y.; Fu, X.; Tang, J.; Jiang, N.; Han, Y.; et al. Reactive Oxygen Species Scavenging Functional Hydrogel Delivers Procyanidins for the Treatment of Traumatic Brain Injury in Mice. ACS Appl. Mater. Interfaces 2022, 14, 33756–33767. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, F.; Yu, J.; Ge, J.; Sun, Y.; Fu, H.; Cheng, Y. Plasmodium vivax Protein PvTRAg23 Triggers Spleen Fibroblasts for Inflammatory Profile and Reduces Type I Collagen Secretion via NF-κBp65 Pathway. Front. Immunol. 2022, 13, 877122. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and their roles in human cancer. Anat. Rec. 2020, 303, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.C.; Fan, P.C.; Tsai, S.Y.; Shih, I.C.; Chiang, H.M. Ixora parviflora Protects against UVB-Induced Photoaging by Inhibiting the Expression of MMPs, MAP Kinases, and COX-2 and by Promoting Type I Procollagen Synthesis. Evid. Based Complement. Altern. Med. 2012, 417346. [Google Scholar]
- Heo, H.; Lee, H.; Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective Activity and Underlying Mechanism of Ginseng Seeds against UVB-Induced Damage in Human Fibroblasts. Antioxidants 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xing, Y.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants 2022, 11, 1900. https://doi.org/10.3390/antiox11101900
Liu X, Xing Y, Yuen M, Yuen T, Yuen H, Peng Q. Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants. 2022; 11(10):1900. https://doi.org/10.3390/antiox11101900
Chicago/Turabian StyleLiu, Xinying, Yi Xing, Michael Yuen, Tina Yuen, Hywel Yuen, and Qiang Peng. 2022. "Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts" Antioxidants 11, no. 10: 1900. https://doi.org/10.3390/antiox11101900
APA StyleLiu, X., Xing, Y., Yuen, M., Yuen, T., Yuen, H., & Peng, Q. (2022). Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants, 11(10), 1900. https://doi.org/10.3390/antiox11101900