Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function
Abstract
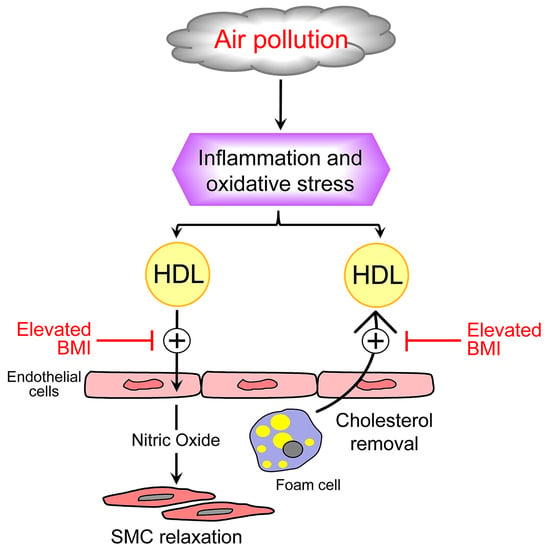
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Collection of Personal Data and Biological Samples
2.3. Exposure Assessment
2.4. Biochemical Analysis
2.5. Nitric Oxide Production
2.6. Macrophage Cholesterol Mass
2.7. Statistical Analysis
3. Results
3.1. Anthropometric, Biochemical, and Clinical Features of Enrolled Subjects
3.2. Production of NO by Endothelial Cells
3.3. Macrophage Cholesterol Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: The Paul Wood Lecture, British Cardiovascular Society 2021. Heart 2022, 108, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Hu, D.; Li, L.; Cui, L.; Xu, J.; Wang, W.; Deng, F.; Guo, X. Joint effect of multiple air pollutants on lipid profiles in obese and normal-weight young adults: The key role of ozone. Environ. Pollut. 2022, 292 Pt A, 118247. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Rossoni, G.; Franceschini, G. Synthetic high density lipoproteins for the treatment of myocardial ischemia/reperfusion injury. Pharmacol. Ther. 2006, 111, 836–854. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Pavanello, C.; Giorgio, E.; Calabresi, L.; Gomaraschi, M. Dysfunctional HDL as a Therapeutic Target for Atherosclerosis Prevention. Curr. Med. Chem. 2019, 26, 1610–1630. [Google Scholar] [CrossRef]
- Holme, S.A.N.; Sigsgaard, T.; Holme, J.A.; Holst, G.J. Effects of particulate matter on atherosclerosis: A link via high-density lipoprotein (HDL) functionality? Part. Fibre Toxicol. 2020, 17, 36. [Google Scholar] [CrossRef]
- Mills, N.L.; Törnqvist, H.; Robinson, S.D.; Gonzalez, M.; Darnley, K.; MacNee, W.; Boon, N.A.; Donaldson, K.; Blomberg, A.; Sandstrom, T.; et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005, 112, 3930–3936. [Google Scholar] [CrossRef]
- Bollati, V.; Iodice, S.; Favero, C.; Angelici, L.; Albetti, B.; Cacace, R.; Cantone, L.; Carugno, M.; Cavalleri, T.; De Giorgio, B.; et al. Susceptibility to particle health effects, miRNA and exosomes: Rationale and study protocol of the SPHERE study. BMC Public Health 2014, 14, 1137. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Analitis, A.; Katsouyanni, K.; Biggeri, A.; Baccini, M.; Forsberg, B.; Bisanti, L.; Kirchmayer, U.; Ballester, F.; Cadum, E.; Goodman, P.; et al. Effects of cold weather on mortality: Results from 15 European cities within the PHEWE project. Am. J. Epidemiol. 2008, 168, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Pergoli, L.; Cantone, L.; Favero, C.; Angelici, L.; Iodice, S.; Pinatel, E.; Hoxha, M.; Dioni, L.; Letizia, M.; Albetti, B.; et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Bonzini, M.; Pergoli, L.; Cantone, L.; Hoxha, M.; Spinazzè, A.; Del Buono, L.; Favero, C.; Carugno, M.; Angelici, L.; Broggi, L.; et al. Short-term particulate matter exposure induces extracellular vesicle release in overweight subjects. Environ. Res. 2017, 155, 228–234. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Dioni, L.; Hoxha, M.; Fedeli, U.; Mielzynska-Svach, D.; Baccarelli, A.A. Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, L.; Ossoli, A.; Giorgio, E.; Pisciotta, L.; Lucchi, T.; Grigore, L.; Pavanello, C.; Granata, A.; Pasta, A.; Arosio, B.; et al. LIPA gene mutations affect the composition of lipoproteins: Enrichment in ACAT-derived cholesteryl esters. Atherosclerosis 2020, 297, 8–15. [Google Scholar] [CrossRef]
- Calabresi, L.; Nilsson, P.; Pinotti, E.; Gomaraschi, M.; Favari, E.; Adorni, M.P.; Bernini, F.; Sirtori, C.R.; Calandra, S.; Franceschini, G.; et al. A novel homozygous mutation in CETP gene as a cause of CETP deficiency in a Caucasian kindred. Atherosclerosis 2009, 205, 506–511. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arter. Thromb. Vasc. Biol. 2019, 39, 513–522. [Google Scholar] [CrossRef]
- Mathew, A.V.; Yu, J.; Guo, Y.; Byun, J.; Chen, Y.E.; Wang, L.; Liu, M.; Bard, R.L.; Morishita, M.; Huang, W.; et al. Effect of Ambient Fine Particulate Matter Air Pollution and Colder Outdoor Temperatures on High-Density Lipoprotein Function. Am. J. Cardiol. 2018, 122, 565–570. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Franceschini, G. Endothelial protection by high-density lipoproteins: From bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Wauters, A.; Dreyfuss, C.; Pochet, S.; Hendrick, P.; Berkenboom, G.; van de Borne, P.; Argacha, J.F. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 2013, 62, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Lucking, A.J.; Lundbäck, M.; Barath, S.L.; Mills, N.L.; Sidhu, M.K.; Langrish, J.P.; Boon, N.A.; Pourazar, J.; Badimon, J.J.; Gerlofs-Nijland, M.E.; et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 2011, 123, 1721–1728. [Google Scholar] [CrossRef]
- Rankin, G.D.; Kabéle, M.; Brown, R.; Macefield, V.G.; Sandström, T.; Bosson, J.A. Acute Exposure to Diesel Exhaust Increases Muscle Sympathetic Nerve Activity in Humans. J. Am. Heart Assoc. 2021, 10, e018448. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, J.G.; Rissler, J.; Lykkesfeldt, J.; Sallsten, G.; Kristiansen, J.; Møller, P.P.; Loft, S. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part. Fibre Toxicol. 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.H.; Bae, S.; Park, H.Y.; Hong, Y.C. eNOS gene polymorphisms modify the association of PM10 with oxidative stress. Toxicol. Lett. 2012, 214, 263–267. [Google Scholar] [CrossRef]


| All | BMI 18.5–24.9 | BMI 25–29.9 | BMI ≥ 30 | p-Value | |
|---|---|---|---|---|---|
| (n = 91) | (n = 23) | (n = 26) | (n = 42) | ||
| Age, years | 52.1 ± 9.6 | 45.0 ± 5.6 | 52.3 ± 8.8 | 55.6 ± 9.8 | <0.0001 |
| Gender | |||||
| Males | 27 (29.67%) | 4 (17.39%) | 13 (50.00%) | 10 (23.81%) | 0.0306 |
| Females | 64 (70.33%) | 19 (82.61%) | 13 (50.00%) | 32 (76.19%) | |
| BMI, kg/m2 | 28.9 ± 5.0 | 21.8 ± 1.9 | 28.0 ± 1.6 | 33.3 ± 1.6 | - |
| Smoking status | |||||
| Never smoker | 49 (53.85%) | 17 (73.91%) | 11 (42.31%) | 21 (50.00%) | 0.0739 |
| Former smoker | 26 (28.57%) | 3 (13.04%) | 12 (46.15%) | 11 (26.19%) | |
| Current smoker | 16 (17.58%) | 3 (13.04%) | 3 (11.54%) | 10 (23.81%) | |
| Education | |||||
| Primary school | 3 (3.30%) | 0 (0.0%) | 1 (3.85%) | 2 (4.76%) | <0.0001 |
| Secondary school | 16 (17.58%) | 0 (0.0%) | 2 (7.69%) | 14 (33.33%) | |
| High school | 35 (38.46%) | 5 (21.74%) | 9 (34.62%) | 21 (50.00%) | |
| University or more | 37 (40.66%) | 18 (78.26%) | 14 (53.85%) | 5 (11.9%) | |
| Occupation | |||||
| Employee | 72 (79.12%) | 23 (100.0%) | 21 (80.77%) | 28 (66.67%) | 0.0189 |
| Unemployed | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Pensioner | 14 (15.38%) | 0 (0.0%) | 4 (15.38%) | 10 (23.81%) | |
| Housewife | 5 (5.49%) | 0 (0.0%) | 1 (3.85%) | 4 (9.52%) | |
| Total cholesterol, mg/dL | 212.6 ± 43.2 | 213.8 ± 33.1 | 211.7 ± 44.4 | 213.0 ± 47.6 | 0.9368 |
| HDL cholesterol, mg/dL | 56.9 ± 17.6 | 68.0 ± 16.5 | 59.0 ± 16.3 | 49.6 ± 15.6 | 0.0001 |
| LDL cholesterol, mg/dL | 133.3 ± 36.9 | 126.3 ± 27 | 129.7 ± 34.8 | 139.9 ± 41.7 | 0.2664 |
| Triglycerides, mg/dL | 113 [77;162] | 83.2 [69.7;116.1] | 93.4 [73;142.7] | 145.5 [109;215] | 0.0001 |
| Interleukin-8, pg/mL | 6.7 ± 6.83 | 7.6 ± 6.1 | 8.4 ± 11.9 | 5.1 ± 4.8 | 0.2500 |
| N. of subjects with IL-8 < LLOQ | 25 (27.47%) | 0 (0.0%) | 5 (19.23%) | 20 (48.78%) | - |
| Mitochondrial DNA, cn | 1.08 ± 0.31 | 1.00 ± 0.23 | 1.13 ± 0.39 | 1.08 ± 0.29 | 0.3358 |
| Diabetes | |||||
| Yes | 9 (9.89%) | 0 (0.0%) | 2 (7.69%) | 7 (16.67%) | 0.0930 |
| No | 82 (90.11%) | 23 (100.0%) | 24 (92.31%) | 35 (83.33%) | |
| Hypertension | |||||
| Yes | 40 (43.96%) | 1 (4.35%) | 9 (34.62%) | 30 (71.43%) | <0.0001 |
| No | 51 (56.04%) | 22 (95.65%) | 17 (65.38%) | 12 (28.57%) | |
| Mean ± SD | |
|---|---|
| PM10 exposure (µg/m3) | |
| Day 0 | 50.61 ± 22.49 |
| Day -1 | 48.35 ± 22.01 |
| Day -2 | 47.36 ± 22.02 |
| Day -3 | 51.61 ± 27.68 |
| Day -4 | 50.67 ± 24.00 |
| Day -5 | 53.09 ± 22.97 |
| Day -6 | 49.72 ± 24.41 |
| PM2.5 exposure (µg/m3) | |
| Day 0 | 38.19 ± 20.82 |
| Day -1 | 36.23 ± 18.47 |
| Day -2 | 37.97 ± 19.49 |
| Day -3 | 34.37 ± 17.93 |
| Day -4 | 37.51 ± 17.38 |
| Day -5 | 41.22 ± 18.59 |
| Day -6 | 38.92 ± 20.58 |
| Apparent temperature (°C) Day 0 | 7.37 ± 4.81 |
| NO Production | Cholesterol Mass | |||||
|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |
| PM10 exposure | ||||||
| Day 0 | 0.016 | 0.014 | 0.2757 | −0.014 | 0.016 | 0.3909 |
| Day -1 | 0.021 | 0.014 | 0.1411 | −0.016 | 0.016 | 0.3320 |
| Day -2 | 0.003 | 0.014 | 0.8424 | 0.004 | 0.016 | 0.8103 |
| Day -3 | −0.002 | 0.011 | 0.8590 | 0.011 | 0.012 | 0.3721 |
| Day -4 | −0.004 | 0.013 | 0.7667 | 0.021 | 0.014 | 0.1421 |
| Day -5 | 0.004 | 0.013 | 0.7536 | 0.009 | 0.015 | 0.5560 |
| Day -6 | 0.006 | 0.013 | 0.6399 | 0.007 | 0.014 | 0.6001 |
| PM2.5 exposure | ||||||
| Day 0 | 0.007 | 0.015 | 0.6322 | −0.027 | 0.017 | 0.1082 |
| Day -1 | 0.025 | 0.017 | 0.1468 | −0.034 | 0.020 | 0.0916 |
| Day -2 | 0.020 | 0.016 | 0.2119 | −0.008 | 0.018 | 0.6843 |
| Day -3 | 0.008 | 0.017 | 0.6329 | −0.005 | 0.019 | 0.8068 |
| Day -4 | 0.008 | 0.018 | 0.6726 | 0.002 | 0.020 | 0.9254 |
| Day -5 | 0.008 | 0.018 | 0.6672 | −0.011 | 0.020 | 0.5759 |
| Day -6 | 0.030 | 0.017 | 0.0815 | −0.002 | 0.020 | 0.9037 |
| Independent Variable | BMI | β | SE | p-Value | p-Value for Interaction |
|---|---|---|---|---|---|
| PM10 Day -1 | Normal weight: 21.25 kg/m2 | −0.0650 | 0.0301 | 0.0342 | 0.0583 |
| Overweight: 27.5 kg/m2 | −0.0270 | 0.0170 | 0.1191 | ||
| Obesity: 32.5 kg/m2 | 0.0067 | 0.0197 | 0.7365 | ||
| PM2.5 Day -1 | Normal weight: 21.25 kg/m2 | −0.0560 | 0.0368 | 0.1309 | 0.4724 |
| Overweight: 27.5 kg/m2 | −0.0390 | 0.0211 | 0.0687 | ||
| Obesity: 32.5 kg/m2 | −0.0240 | 0.0241 | 0.3211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossoli, A.; Favero, C.; Vigna, L.; Pesatori, A.C.; Bollati, V.; Gomaraschi, M. Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function. Antioxidants 2022, 11, 1938. https://doi.org/10.3390/antiox11101938
Ossoli A, Favero C, Vigna L, Pesatori AC, Bollati V, Gomaraschi M. Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function. Antioxidants. 2022; 11(10):1938. https://doi.org/10.3390/antiox11101938
Chicago/Turabian StyleOssoli, Alice, Chiara Favero, Luisella Vigna, Angela Cecilia Pesatori, Valentina Bollati, and Monica Gomaraschi. 2022. "Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function" Antioxidants 11, no. 10: 1938. https://doi.org/10.3390/antiox11101938
APA StyleOssoli, A., Favero, C., Vigna, L., Pesatori, A. C., Bollati, V., & Gomaraschi, M. (2022). Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function. Antioxidants, 11(10), 1938. https://doi.org/10.3390/antiox11101938