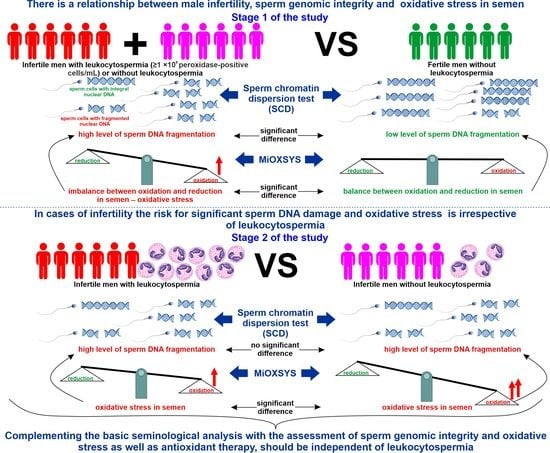
Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Basic Semen Analysis
2.3. Sperm Chromatin Dispersion (SCD) Test
2.4. Static oxidation-reduction Potential (sORP) in Semen
2.5. Statistical Analysis
3. Results
3.1. Infertile vs. Fertile Men
3.2. Infertile Leukocytospermic Men vs. Infertile Nonleukocytospermic Men
3.3. Correlations between Study Parameters
4. Discussion
4.1. Relationships between Male Infertility, SDF, and sORP
4.2. Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Can Occur Irrespective of Leukocytospermia
5. Conclusions
6. Limitations of this Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male Infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Agarwal, A.; Farkouh, A.; Parekh, N.; Zini, A.; Arafa, M.; Kandil, H.; Tadros, N.; Busetto, G.M.; Ambar, R.; Parekattil, S.; et al. Sperm DNA Fragmentation: A Critical Assessment of Clinical Practice Guidelines. World J. Men’s Health 2022, 40, 30–37. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Agarwal, A. Comparative Analysis of Tests Used to Assess Sperm Chromatin Integrity and DNA Fragmentation. Andrologia 2021, 53, e13718. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. IJMS 2021, 22, 10043. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA Fragmentation Testing: Summary Evidence and Clinical Practice Recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of Sperm DNA Damage and Its Impact on Male Infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative Stress and Male Infertility: Current Knowledge of Pathophysiology and Role of Antioxidant Therapy in Disease Management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Brunner, R.J.; Demeter, J.H.; Sindhwani, P. Review of Guidelines for the Evaluation and Treatment of Leukocytospermia in Male Infertility. World J. Men’s Health 2019, 37, 128. [Google Scholar] [CrossRef]
- Henkel, R.; Offor, U.; Fisher, D. The Role of Infections and Leukocytes in Male Infertility. Andrologia 2021, 53, e13743. [Google Scholar] [CrossRef]
- Liu, K.-S.; Mao, X.-D.; Pan, F.; Chen, Y.-J. Application of Leukocyte Subsets and Sperm DNA Fragment Rate in Infertile Men with Asymptomatic Infection of Genital Tract. Ann. Palliat. Med. 2021, 10, 1021. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Zhang, Y.-J.; Wang, S.; Wei, Y.; Li, F.; Feng, K.-J. The Effect of Screening and Treatment of Ureaplasma Urealyticum Infection on Semen Parameters in Asymptomatic Leukocytospermia: A Case—Control Study. BMC Urol. 2020, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Pellati, D.; Mylonakis, I.; Bertoloni, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Armanini, D. Genital Tract Infections and Infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 3–11. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Oxidative Stress, Spermatozoa and Leukocytic Infiltration: Relationships Forged by the Opposing Forces of Microbial Invasion and the Search for Perfection. J. Reprod. Immunol. 2013, 100, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive Oxygen Species in Male Reproduction: A Boon or a Bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male Subfertility and Oxidative Stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative Stress and Male Infertility. Reprod. Med. Biol. 2021, 20, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.R. Leukocytes and Oxidative Stress: Dilemma for Sperm Function and Male Fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, D.; Ohlander, S.; Niederberger, C. Pyospermia: Background and Controversies. FS Rep. 2021, 2, 2–6. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of Bacterial Infection on Sperm Quality and DNA Fragmentation in Subfertile Men with Leukocytospermia. BMC Mol. Cell Biol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Pratap, H.; Hottigoudar, S.; Nichanahalli, K.; Rajendran, S.; Bheemanathi, H. Sperm DNA Integrity in Leukocytospermia and Its Association with Seminal Adenosine Deaminase. J. Hum. Reprod. Sci. 2019, 12, 182. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of Sperm DNA and Male Infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Ko, E.Y. Oxidative Stress in the Pathophysiology of Male Infertility. Andrologia 2021, 53, e13581. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The Effect of Bacteriospermia and Leukocytospermia on Conventional and Nonconventional Semen Parameters in Healthy Young Normozoospermic Males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Mechanisms of the Harmful Effects of Bacterial Semen Infection on Ejaculated Human Spermatozoa: Potential Inflammatory Markers in Semen. Folia Histochem. Cytobiol. 2015, 53, 201–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraczek, M.; Szumala-Kakol, A.; Dworacki, G.; Sanocka, D.; Kurpisz, M. In Vitro Reconstruction of Inflammatory Reaction in Human Semen: Effect on Sperm DNA Fragmentation. J. Reprod. Immunol. 2013, 100, 76–85. [Google Scholar] [CrossRef]
- Fraczek, M.; Sanocka, D.; Kamieniczna, M.; Kurpisz, M. Proinflammatory Cytokines as an Intermediate Factor Enhancing Lipid Sperm Membrane Peroxidation in In Vitro Conditions. J. Androl. 2008, 29, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Barbăroșie, C.; Agarwal, A.; Henkel, R. Diagnostic Value of Advanced Semen Analysis in Evaluation of Male Infertility. Andrologia 2021, 53, e13625. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Pang, M.-G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Piasecka, M.; Kawiak, J. Sperm Mitochondria of Patients with Normal Sperm Motility and with Asthenozoospermia: Morphological and Functional Study. Folia Histochem. Cytobiol. 2003, 41, 125–139. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization Press: Geneva, Switzerland, 2010. [Google Scholar]
- Gill, K.; Rosiak-Gill, A.; Jakubik, J.; Patorski, L.; Lukaszuk, M.; Piasecka, M. The Higher Risk for Sperm DNA Damage in Infertile Men. Ginekol. Pol. 2019, 90, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Gill, K.; Jakubik, J.; Kups, M.; Rosiak-Gill, A.; Kurzawa, R.; Kurpisz, M.; Fraczek, M.; Piasecka, M. The Impact of Sedentary Work on Sperm Nuclear DNA Integrity. Folia Histochem. Cytobiol. 2019, 57, 15–22. [Google Scholar] [CrossRef]
- Gill, K.; Jakubik, J.; Rosiak-Gill, A.; Kups, M.; Lukaszuk, M.; Kurpisz, M.; Fraczek, M.; Piasecka, M. Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential. Int. J. Environ. Res. Public Health 2019, 16, 2004. [Google Scholar] [CrossRef] [Green Version]
- Gill, K.; Kups, M.; Harasny, P.; Machalowski, T.; Grabowska, M.; Lukaszuk, M.; Matuszewski, M.; Duchnik, E.; Fraczek, M.; Kurpisz, M.; et al. The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen. Int. J. Environ. Res. Public Health 2021, 18, 5977. [Google Scholar] [CrossRef]
- Jakubik-Uljasz, J.; Gill, K.; Rosiak-Gill, A.; Piasecka, M. Relationship between Sperm Morphology and Sperm DNA Dispersion. Transl. Androl. Urol. 2020, 9, 405–415. [Google Scholar] [CrossRef]
- Kups, M.; Gill, K.; Rosiak-Gill, A.; Harasny, P.; Machalowski, T.; Grabowska, M.; Kurzawa, R.; Sipak, O.; Piasecka, M. Evaluation of Selected Semen Parameters and Biomarkers of Male Infertility—Preliminary Study. F1000Res 2022, 11, 591. [Google Scholar] [CrossRef]
- Joao, F.; Duval, C.; Bélanger, M.-C.; Lamoureux, J.; Xiao, C.W.; Ates, S.; Benkhalifa, M.; Miron, P. Reassessing the Interpretation of Oxidation-Reduction Potential in Male Infertility. Reprod. Fertil. 2022, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Castleton, P.E.; Deluao, J.C.; Sharkey, D.J.; McPherson, N.O. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Impact of Oxidative Stress on Male and Female Germ Cells: Implications for Fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, T.M.; Aziz, N.; Sharma, R.K.; Lewis-Jones, I.; Thomas, A.J.; Agarwal, A. Novel Association between Sperm Deformity Index and Oxidative Stress-Induced DNA Damage in Infertile Male Patients. Asian J. Androl. 2005, 7, 121–126. [Google Scholar] [CrossRef]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J.; Agarwal, A. Novel Association between Sperm Reactive Oxygen Species Production, Sperm Morphological Defects, and the Sperm Deformity Index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef]
- Arafa, M.; Agarwal, A.; Al Said, S.; Majzoub, A.; Sharma, R.; Bjugstad, K.B.; AlRumaihi, K.; Elbardisi, H. Semen Quality and Infertility Status Can Be Identified through Measures of Oxidation-Reduction Potential. Andrologia 2018, 50, e12881. [Google Scholar] [CrossRef]
- Cicek, O.S.Y.; Kaya, G.; Alyuruk, B.; Doger, E.; Girisen, T.; Filiz, S. The Association of Seminal Oxidation Reduction Potential with Sperm Parameters in Patients with Unexplained and Male Factor Infertility. Int. Braz. J. Urol. 2021, 47, 112–119. [Google Scholar] [CrossRef]
- Majzoub, A.; Arafa, M.; El Ansari, W.; Mahdi, M.; Agarwal, A.; Al-Said, S.; Elbardisi, H. Correlation of Oxidation Reduction Potential and Total Motile Sperm Count: Its Utility in the Evaluation of Male Fertility Potential. Asian J. Androl. 2020, 22, 317. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.; Henkel, R.; Agarwal, A.; Majzoub, A.; Elbardisi, H. Correlation of Oxidation-Reduction Potential with Hormones, Semen Parameters and Testicular Volume. Andrologia 2019, 51, e13258. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic Application of Oxidation-Reduction Potential Assay for Measurement of Oxidative Stress: Clinical Utility in Male Factor Infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbaki, S.A.; Sabry, J.H.; Al-Adl, A.M.; Sabry, H.H. The Impact of Coexisting Sperm DNA Fragmentation and Seminal Oxidative Stress on the Outcome of Varicocelectomy in Infertile Patients: A Prospective Controlled Study. Arab J. Urol. 2017, 15, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of Sperm DNA Fragmentation with Lifestyle Factors and Semen Parameters of Saudi Men and Its Impact on ICSI Outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Han, M.; Ding, J.; Wang, F.; Wang, G.; Shen, L.; Wang, J.; Zheng, B.; Meng, Q.; Wang, W.; et al. Importance of a Semen Analysis Report for Determining the Relationship between SCSA Sperm DNA Fragmentation Index and Assisted Reproductive Technology Pregnancy Rate. Reprod. Biol. 2020, 20, 460–464. [Google Scholar] [CrossRef]
- Khalafalla, K.; Majzoub, A.; Elbardisi, H.; Bhathella, A.; Chaudhari, A.; Agarwal, A.; Henkel, R.; AlMarzooki, T.; Burjaq, H.; Arafa, M. The Effect of Sperm DNA Fragmentation on Intracytoplasmic Sperm Injection Outcome. Andrologia 2021, 53, e14180. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Trieu, T.S.; Tran, T.O.; Luong, T.L.A. Evaluation of Sperm DNA Fragmentation Index, Zinc Concentration and Seminal Parameters from Infertile Men with Varicocele. Andrologia 2019, 51, e13184. [Google Scholar] [CrossRef]
- Vinnakota, C.; Cree, L.; Peek, J.; Morbeck, D.E. Incidence of High Sperm DNA Fragmentation in a Targeted Population of Subfertile Men. Syst. Biol. Reprod. Med. 2019, 65, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Li, G.; Jin, H.; Guo, Y.; Sun, Y. The Effect of Sperm DNA Fragmentation Index on Assisted Reproductive Technology Outcomes and Its Relationship with Semen Parameters and Lifestyle. Transl. Androl. Urol. 2019, 8, 356–365. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization Press: Geneva, Switzerland, 2021. [Google Scholar]
- Agarwal, A.; Henkel, R.; Sharma, R.; Tadros, N.N.; Sabanegh, E. Determination of Seminal Oxidation-Reduction Potential (ORP) as an Easy and Cost-Effective Clinical Marker of Male Infertility. Andrologia 2018, 50, e12914. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.; Ahmad, G.; Khan, H.L.; Ayaz, A.; Ali, A.H.A. Impact of Single and Multiple Sperm Abnormalities and Low-Level Leukocytospermia on Sperm DNA. J. Men’s Health 2019, 15, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Arafa, M.; Henkel, R.; Agarwal, A.; Robert, K.; Finelli, R.; Majzoub, A.; ElBardisi, H. Seminal Oxidation–Reduction Potential Levels Are Not Influenced by the Presence of Leucocytospermia. Andrologia 2020, 52, e13609. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zeng, R.; Yang, Z.; Xu, L.; Ma, Q.; Yang, Y.; Bai, Y.; Yang, Y.; Bai, P. Effects of Leukocytospermia on the Outcomes of Assisted Reproductive Technology. Andrologia 2022, 54, e14403. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; D’Andrea, S.; Martorella, A.; Minaldi, E.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship between Leukocytospermia, Reproductive Potential after Assisted Reproductive Technology, and Sperm Parameters: A Systematic Review and Meta-analysis of Case–Control Studies. Andrologia 2020, 8, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, A.; Sayed, H.; Mosaad, E.; Mahmoud, A.; Farag, F.; Taha, E.A. Impact of Leukocytospermia on Sperm Dynamic Motility Parameters, DNA and Chromosomal Integrity. Cent. Eur. J. Urol. 2018, 71, 470–475. [Google Scholar] [CrossRef]
- Fariello, R.M.; Del Giudice, P.T.; Spaine, D.M.; Fraietta, R.; Bertolla, R.P.; Cedenho, A.P. Effect of Leukocytospermia and Processing by Discontinuous Density Gradient on Sperm Nuclear DNA Fragmentation and Mitochondrial Activity. J. Assist. Reprod. Genet. 2009, 26, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Erenpreiss, J.; Hlevicka, S.; Zalkalns, J.; Erenpreisa, J. Effect of Leukocytospermia on Sperm DNA Integrity: A Negative Effect in Abnormal Semen Samples. J. Androl. 2002, 23, 717–723. [Google Scholar]
- García Rodríguez, A.; de la Casa, M.; Johnston, S.; Gosálvez, J.; Roy, R. Association of Polymorphisms in Genes Coding for Antioxidant Enzymes and Human Male Infertility. Ann. Hum. Genet. 2019, 83, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total Antioxidant Capacity-Relevance, Methods and Clinical Implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric Oxide, 8-Hydroxydeoxyguanosine, and Total Antioxidant Capacity in Human Seminal Plasma of Infertile Men and Their Relationship with Sperm Parameters. Clin. Exp. Reprod. Med. 2020, 47, 54–60. [Google Scholar] [CrossRef]
- Fraczek, M.; Wojnar, L.; Kamieniczna, M.; Piasecka, M.; Gill, K.; Kups, M.; Chopyak, V.; Havrylyuk, A.; Nakonechnyy, J.; Nakonechnyy, A.; et al. Seminal Plasma Analysis of Oxidative Stress in Different Genitourinary Topographical Regions Involved in Reproductive Tract Disorders Associated with Genital Heat Stress. Int. J. Mol. Sci. 2020, 21, 6427. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Lewandowska, A.; Budzinska, M.; Kamieniczna, M.; Wojnar, L.; Gill, K.; Piasecka, M.; Kups, M.; Havrylyuk, A.; Chopyak, V.; et al. The Role of Seminal Oxidative Stress Scavenging System in the Pathogenesis of Sperm DNA Damage in Men Exposed and Not Exposed to Genital Heat Stress. Int. J. Environ. Res. Public Health 2022, 19, 2713. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Ravichandran, A.; Thiagarajan, N.; Govindarajan, M.; Dhandayuthapani, S.; Suresh, S. Seminal Reactive Oxygen Species and Total Antioxidant Capacity: Correlations with Sperm Parameters and Impact on Male Infertility. Clin. Exp. Reprod. Med. 2018, 45, 88–93. [Google Scholar] [CrossRef]


| Sperm Category | Phenotype of Sperm Cells Head | Description |
|---|---|---|
| Sperm cells with non-fragmented nuclear DNA | Head with big halo | Halo equal to or higher than the diameter of the core of spermatozoa |
| Head with medium halo | Halo > 1/3 of the diameter of the core of spermatozoa | |
| Head with small halo | Halo ≤ 1/3 of the diameter of the core of spermatozoa | |
| Sperm cells with fragmented nuclear DNA | Head without halo | Absence of halo but strongly stained core |
| Head with no halo and degraded chromatin | Absence of halo and simultaneously an irregularly or weakly stained core |
| Parameters | Total (n = 204) | Group 1: Infertile Men with Leukocytospermia (n = 47) | Group 2: Infertile Men without Leukocytospermia (n = 77) | Group 3: Fertile Men without Leukocytospermia (n = 80) | p 1 vs. 3 | p 2 vs. 3 | p 1 vs. 2 |
|---|---|---|---|---|---|---|---|
| Median (Range) Mean ± SD | |||||||
| Age (y) | 33.00 (22.00–49.00) 33.00 ± 4.80 | 34.00 (27.00–46.00) 34.17 ± 4.37 | 33.00 (25.00–49.00) 33.55 ± 4.95 | 32.00 (22.00–47.00) 31.78 ± 4.68 | 0.015445 | NS | NS |
| Semen volume (mL) | 3.00 (0.50–8.00) 3.21 ± 1.59 | 3.00 (0.50–8.00) 3.20 ± 1.79 | 3.00 (0.50–6.50) 3.04 ± 1.41 | 3.00 (0.50–8.00) 3.37 ± 1.64 | NS | NS | NS |
| Sperm concentration (×106/mL) | 23.08 (0.05–210.00) 34.62 ± 37.12 | 16.75 (0.44–130.00) 25.15 ± 26.27 | 7.00 (0.05–146.50) 18.01 ± 25.47 | 50.50 (4.80–210.00) 56.16 ± 41.50 | 0.000008 | <0.000001 | NS |
| Total number of spermatozoa (×106) | 63.91 (0.25–840.00) 108.68 ± 137.68 | 52.25 (0.50–575.00) 74.22 ± 91.04 | 21.30 (0.25–365.75) 50.25 ± 68.86 | 139.41 (21.60–840.00) 185.16 ± 171.27 | 0.000008 | <0.000001 | NS |
| Morphologically normal spermatozoa (%) | 2.00 (0.00–13.00) 2.47 ± 3.07 | 0.00 (0.00–10.00) 1.38 ± 2.38 | 0.00 (0.00–9.00) 1.12 ± 1.77 | 4.00 (0.00–13.00) 4.41 ± 3.42 | <0.000001 | <0.000001 | NS |
| TZI | 1.75 (1.15–2.58) 1.78 ± 0.29 | 1.81 (1.46–2.58) 1.84 ± 0.28 | 1.89 (1.36–2.50) 1.90 ± 0.30 | 1.64 (1.15–2.25) 1.62 ± 0.22 | 0.000391 | <0.000001 | NS |
| Total sperm head defects (%) | 96.00 (72.00–100.00) 94.75 ± 5.13 | 98.00 (72.00–100.00) 95.93 ± 5.71 | 98.00 (87.00–100.00) 94.81 ± 3.71 | 92.00 (92.00–100.00) 94.75 ± 5.13 | 0.000005 | < 0.000001 | NS |
| Total sperm midpieces defects (%) | 41.50 (9.00–84.00) 42.74 ± 5.13 | 42.00 (20.00–78.00) 43.80 ± 15.06 | 51.00 (9.00–84.00) 50.10 ± 17.18 | 36.50 (9.00–73.00) 35.03 ± 13.75 | 0.020289 | < 0.000001 | NS |
| Total sperm tail defects (%) | 29.00 (6.00–88.00) 31.91 ± 14.90 | 30.00 (6.00–88.00) 34.36 ± 17.56 | 37.00 (8.00–65.00) 36.32 ± 15.02 | 25.00 (6.00–50.00) 26.23 ± 10.92 | 0.030531 | 0.000075 | NS |
| Immature sperm with excess residual cytoplasm (%) | n = 203 4.00 (0.00–60.00) 4.99 ± 6.36 | n = 46 6.50 (0.00–41.00) 7.21 ± 6.43 | 4.00 (0.00–60.00) 6.16 ± 8.10 | 2.00 (0.00–8.00) 2.57 ± 2.62 | <0.000001 | 0.000281 | NS |
| Progressive motility (%) | 51.00 (0.00–90.00) 46.91 ± 23.14 | 39.00 (0.00–74.00) 39.25 ± 22.17 | 33.00 (0.00–79.00) 34.46 ± 21.03 | 67.00 (22.00–90.00) 63.40 ± 14.25 | <0.000001 | <0.000001 | NS |
| Nonprogressive motility (%) | 6.00 (0.00–33.00) 7.38 ± 6.42 | 6.00 (0.00–16.00) 6.10 ± 3.80 | 5.00 (0.00–16.00) 5.24 ± 3.64 | 8.00 (0.00–33.00) 10.20 ± 8.45 | NS | 0.001066 | NS |
| Eosin-negative spermatozoa—live cells (%) | 77.00 (3.00–96.00) 75.15 ± 13.56 | 74.00 (48.00–90.00) 73.25 ± 10.34 | 74.00 (3.00–96.00) 69.16 ± 16.46 | 84.00 (62.00–95.00) 82.02 ± 8.06 | 0.000021 | <0.000001 | NS |
| HOS test-positive spermatozoa—live cells (%) | n = 168 77.00 (13.00–95.00) 74.57 ± 11.65 | n = 38 73.00 (34.00–92.00) 73.34 ± 73.34 | n = 55 71.00 (13.00–90.00) 90.00 ± 69.83 | n = 75 80.00 (58.00–95.00) 95.00 ± 78.68 | 0.034308 | 0.000117 | NS |
| Peroxidase-positive cells (×106/mL) | 0.30 (0.00–27.00) 0.95 ± 2.42 | 1.75 (1.00–27.00) 3.20 ± 4.37 | 0.25 (0.00–0.96) 0.32 ± 0.24 | 0.25 (0.00–0.75) 0.23 ± 0.19 | <0.000001 | NS | 0.0100 |
| Round sperm cells (×106/mL) | n = 203 4.00 (0.00–60.00) 4.99 ± 6.36 | n = 46 6.50 (0.00–41.00) 7.21 ± 6.43 | 4.00 (0.00–60.00) 6.16 ± 8.10 | 2.00 (0.00–8.00) 2.57 ± 2.62 | <0.000001 | 0.042878 | 0.008361 |
| SDF (%) | n = 202 17.00 (3.00–48.00) 18.68 ± 8.89 | n = 46 24.00 (5.00–44.00) 22.10 ± 8.98 | 19.00 (7.00–48.00) 21.05 ± 8.77 | n = 79 13.00 (3.00–34.00) 14.37 ± 7.16 | 0.034308 | 0.000117 | NS |
| Raw ORP (mV) | 45.80 (2.10–184.10) 49.26 ± 29.63 | 49.80 (3.70–175.40) 55.50 ± 37.34 | 56.50 (14.09–121.80) 55.72 ± 26.23 | 35.20 (2.10–184.10) 39.36 ± 26.23 | 0.021902 | 0.00010 | NS |
| sORP (mV/106 sperm cells/mL) | 1.62 (0.02–196.50) 13.22 ± 35.89 | 2.05 (0.09–128.00) 7.12 ± 19.27 | 4.90 (0.28–196.50) 29.75 ± 52.49 | 0.62 (0.02–5.00) 0.91 ± 0.90 | 0.000007 | <0.000001 | 0.004418 |
| Calculated SDF and sORP Threshold | Total %(n) | Group 1: Infertile Men with Leukocytospermia %(n) | Group 2: Infertile Men without Leukocytospermia %(n) | Group 3: Fertile Men without Leukocytospermia %(n) | p 1 vs. 3 | p 2 vs. 3 | p 1 vs. 2 |
|---|---|---|---|---|---|---|---|
| SDF > 13% | n = 202 67.33 (136) | n = 46 82.61 (38) | n = 77 81.81 (63) | n = 79 44.30 (35) | <0.0001 | <0.0001 | NS |
| sORP > 1.40 mV/106 sperm cells/mL | n = 204 53.43 (109) | n = 47 65.95 (31) | n = 77 84.42 (65) | n = 80 16.25 (13) | <0.0001 | <0.0001 | 0.0253 |
| Calculated SDF and sORP Threshold | Total %(n) | Group 1: Infertile Men with Leukocytospermia %(n) | Group 2: Infertile Men without Leukocytospermia %(n) | Group 3: Fertile Men without Leukocytospermia %(n) | OR1 (95% CI) p | OR2 (95% CI) p | OR3 (95% CI) p |
|---|---|---|---|---|---|---|---|
| SDF > 13% | n = 202 67.33 (136) | n = 46 82.61 (38) | n = 77 81.81 (63) | n = 79 44.30 (35) | 5.9714 (2.4713–14.4289) 0.0001 | 5.6571 (2.7271–11.7354) <0.0001 | 0.9474 (0.3637–2.4679) NS |
| sORP > 1.40 mV/106 sperm cells/mL | n = 204 53.43 (109) | n = 47 65.95 (31) | n = 77 84.42 (65) | n = 80 16.25 (13) | 9.9856 (4.2822–23.2853) <0.0001 | 27.9167 (11.8652–65.6830) <0.0001 | 0.3577 (0.1510–0.8471) 0.0194 |
| Parameters | SDF (%) rs(p) | Raw ORP (mV) rs(p) | sORP (mV/106 Sperm Cells/mL) rs(p) |
|---|---|---|---|
| Age (y) | 0.1833 (0.009004) | 0.0547 (NS) | 0.0619 (NS) |
| Semen volume (mL) | 0.0246 (NS) | 0.0333 (NS) | 0.0170 (NS) |
| Sperm concentration (×106/mL) | −0.2777 (0.000063) | −0.2095 (0.002625) | −0.8132 (<0.000001) |
| Total number of spermatozoa (×106) | −0.2590 (0.000198) | −0.2093 (0.002625) | −0.7617 (<0.000001) |
| Morphologically normal spermatozoa (%) | −0.4889 (<0.000001) | −0.1593 (0.022791) | −0.5392 (<0.000001) |
| TZI | 0.2556 (0.000241) | 0.1087 (NS) | 0.5076 (<0.000001) |
| Total sperm head defects (%) | 0.4024 (<0.0000001) | 0.1070 (NS) | 0.4616 (<0.0000001) |
| Total sperm midpieces defects (%) | 0.2570 (0.0002210) | 0.1627 (0.020021) | 0.5232 (<0.0000001) |
| Total sperm tail defects (%) | 0.1576 (0.0250438) | 0.0016 (NS) | 0.3093 (0.0000067) |
| Immature sperm with excess residual cytoplasm (%) | 201 0.1959 (0.0053135) | 203 0.1110 (NS) | 203 0.2956 (0.0000184) |
| Progressive motility (%) | −0.5074 (<0.000001) | −0.1816 (0.009319) | −0.6034 (0.000001) |
| Nonprogressive motility (%) | −0.0244 (NS) | −0.1652 (0.018199) | −0.2698 (0.000095) |
| Eosin-negative spermatozoa—live cells (%) | −0.5044 (<0.000001) | −0.1387 (0.047812 | −0.4852 (<0.000001) |
| HOS test-positive spermatozoa—live cells (%) | n = 166 −0.4341 (<0.000001) | −0.1099 (NS) | n = 168 −0.3315 (0.001457) |
| Peroxidase-positive cells (×106/mL) | 0.2647 (0.000141) | 0.1050 (NS) | 0.1097 (NS) |
| Round sperm cells (×106/mL) | 0.1238 (NS) | 0.0664 (NS) | 0.0638 (NS) |
| SDF (%) | – | 0.2655 (0.000133) | 0.3853 (<0.000001) |
| Raw ORP (mV) | – | 0.5545 (<0.000001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, K.; Machalowski, T.; Harasny, P.; Kups, M.; Grabowska, M.; Duchnik, E.; Sipak, O.; Fraczek, M.; Kurpisz, M.; Kurzawa, R.; et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants 2022, 11, 1987. https://doi.org/10.3390/antiox11101987
Gill K, Machalowski T, Harasny P, Kups M, Grabowska M, Duchnik E, Sipak O, Fraczek M, Kurpisz M, Kurzawa R, et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants. 2022; 11(10):1987. https://doi.org/10.3390/antiox11101987
Chicago/Turabian StyleGill, Kamil, Tomasz Machalowski, Patryk Harasny, Michal Kups, Marta Grabowska, Ewa Duchnik, Olimpia Sipak, Monika Fraczek, Maciej Kurpisz, Rafal Kurzawa, and et al. 2022. "Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia" Antioxidants 11, no. 10: 1987. https://doi.org/10.3390/antiox11101987
APA StyleGill, K., Machalowski, T., Harasny, P., Kups, M., Grabowska, M., Duchnik, E., Sipak, O., Fraczek, M., Kurpisz, M., Kurzawa, R., & Piasecka, M. (2022). Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants, 11(10), 1987. https://doi.org/10.3390/antiox11101987