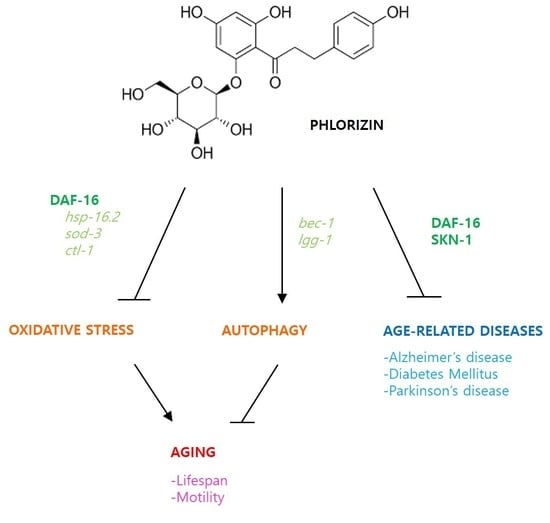
Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Worm Strains and Maintenance
2.2. Resistance to Environmental Stressors
2.3. Intracellular ROS Levels
2.4. Lifespan Assay
2.5. Fertility Assay
2.6. Measurement of Age-Related Decline of Muscle Function
2.7. Expression of Stress-Responsive Genes
2.8. Aβ-Induced Toxicity
2.9. High-Glucose-Diet (HGD)-Induced Toxicity
2.10. Degeneration of Dopaminergic Neurons
2.11. Gene Knockdown by RNAi
2.12. Quantitative RT-PCR
3. Results
3.1. Phlorizin Modulated Response to Environmental Stressors
3.2. Phlorizin Extended Lifespan without Accompanying Reduced Fertility
3.3. Age-Related Decline of Motility Was Delayed by Supplementation with Phlorizin
3.4. Phlorizin Induced Expression of Hsp-16.2 and Sod-3 and Nuclear Localization of DAF-16
3.5. Positive Impact of Phlorizin Was Observed in Age-Related Disease Models
3.6. Lifespan Extension by Phlorizin Was Mediated through Oxidative Stress Response and Autophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Pignolo, R.J. Exceptional Human Longevity. Mayo Clin. Proc. 2019, 94, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span--from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Bartke, A. Healthy aging: Is smaller better?—A mini-review. Gerontology 2012, 58, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S.; Ku, H.H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Xu, P.; Zhang, M.; Chen, J.; Sheng, R.; Ma, Y. Resveratrol-maltol hybrids as multi-target-directed agents for Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 5759–5765. [Google Scholar] [CrossRef] [PubMed]
- Ernst, I.M.; Pallauf, K.; Bendall, J.K.; Paulsen, L.; Nikolai, S.; Huebbe, P.; Roeder, T.; Rimbach, G. Vitamin E supplementation and lifespan in model organisms. Ageing Res. Rev. 2013, 12, 365–375. [Google Scholar] [CrossRef]
- Navarro, A.; Gomez, C.; Sanchez-Pino, M.J.; Gonzalez, H.; Bandez, M.J.; Boveris, A.D.; Boveris, A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1392–R1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlernitzauer, A.; Oiry, C.; Hamad, R.; Galas, S.; Cortade, F.; Chabi, B.; Casas, F.; Pessemesse, L.; Fouret, G.; Feillet-Coudray, C.; et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE 2013, 8, e78788. [Google Scholar]
- Kumar, J.; Park, K.C.; Awasthi, A.; Prasad, B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer’s model. CNS Neurol. Disord. Drug Targets 2015, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, S.H.; Park, S.K. Selenocysteine modulates resistance to environmental stress and confers anti-aging effects in C. elegans. Clinics 2017, 72, 491–498. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, S.K. Supplementation of S-allyl cysteine improves health span in Caenorhabditis elegans. Biosci. J. 2017, 33, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.I.; Park, J.K.; Park, S.K. Lifespan extension and increased resistance to environmental stressors by N-acetyl-L-cysteine in Caenorhabditis elegans. Clinics 2015, 70, 380–386. [Google Scholar] [CrossRef]
- Lee, C.K.; Pugh, T.D.; Klopp, R.G.; Edwards, J.; Allison, D.B.; Weindruch, R.; Prolla, T.A. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic. Biol. Med. 2004, 36, 1043–1057. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.J. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef]
- Zhao, H.; Yakar, S.; Gavrilova, O.; Sun, H.; Zhang, Y.; Kim, H.; Setser, J.; Jou, W.; LeRoith, D. Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes 2004, 53, 2901–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Q.; Lin, Y.; Tan, Z.; Jiang, B.; Xu, L.; Ren, H.; Tai, W.C.; Chan, C.O.; Lee, C.S.; Gu, Z.; et al. Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on alpha-glucosidase in vitro. Food Funct. 2019, 10, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Wang, H.; Wang, M.; Zhao, J.; Wu, Z. Protective effect of apple phlorizin on hydrogen peroxide-induced cell damage in HepG2 cells. J. Food Biochem. 2019, 43, e13052. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci 2021, 22, 962. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Peto, J. Asymptotically efficient rank invariant test procedures. J. R. Statist. Soc. A 1972, 135, 185–207. [Google Scholar] [CrossRef]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Tang, S.Y.; Halliwell, B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2007, 1100, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef] [Green Version]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Blanco, A.; Kim, S.K. Variable pathogenicity determines individual lifespan in Caenorhabditis elegans. PLoS Genet. 2011, 7, e1002047. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.T. The search for DAF-16/FOXO transcriptional targets: Approaches and discoveries. Exp. Gerontol. 2006, 41, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Matilainen, O.; Jin, C.; Glover-Cutter, K.M.; Holmberg, C.I.; Blackwell, T.K. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 2011, 7, e1002119. [Google Scholar] [CrossRef] [PubMed]
- Alcantar-Fernandez, J.; Navarro, R.E.; Salazar-Martinez, A.M.; Perez-Andrade, M.E.; Miranda-Rios, J. Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors. PLoS ONE 2018, 13, e0199888. [Google Scholar] [CrossRef] [Green Version]
- Leonov, A.; Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Lutchman, V.; Medkour, Y.; Titorenko, V.I. Longevity extension by phytochemicals. Molecules 2015, 20, 6544–6572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, J. Effect on human longevity of added dietary chocolate. Nutrition 1998, 14, 869. [Google Scholar]
- Wang, H.; Sun, Z.; Liu, D.; Li, X.; Rehman, R.U.; Wang, H.; Wu, Z. Apple phlorizin attenuates oxidative stress in Drosophila melanogaster. J. Food Biochem. 2019, 43, e12744. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of human LDL oxidation by resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef]
- Chen, H.; Dong, L.; Chen, X.; Ding, C.; Hao, M.; Peng, X.; Zhang, Y.; Zhu, H.; Liu, W. Anti-aging effect of phlorizin on D-galactose-induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 2022, 163, 111769. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Kumar, A.; Jaggi, A.S.; Singh, N. Pharmacological investigations on efficacy of Phlorizin a sodium-glucose co-transporter (SGLT) inhibitor in mouse model of intracerebroventricular streptozotocin induced dementia of AD type. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, G.; Liu, J. Phloretin attenuates behavior deficits and neuroinflammatory response in MPTP induced Parkinson’s disease in mice. Life Sci. 2019, 232, 116600. [Google Scholar] [CrossRef]
- Keowkase, R.; Aboukhatwa, M.; Luo, Y. Fluoxetine protects against amyloid-beta toxicity, in part via daf-16 mediated cell signaling pathway, in Caenorhabditis elegans. Neuropharmacology 2010, 59, 358–365. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X.B.; Wan, Q.L.; Ding, A.J.; Yang, Z.L.; Qiu, M.H.; Sun, H.Y.; Qi, S.H.; Luo, H.R. Otophylloside B Protects Against Abeta Toxicity in Caenorhabditis elegans Models of Alzheimer’s Disease. Nat. Prod. Bioprospect. 2017, 7, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Duan, Z.; Wang, Y.; Wang, M.; Liu, Y.; Wang, X.; Li, H. Protective effect of Terminalia chebula Retz. extract against Abeta aggregation and Abeta-induced toxicity in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 268, 113640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, H.; Dong, J.; Yang, W.; Liu, T.; Wang, Y.; Wang, X.; Wang, M.; Zhi, D. Rose Essential Oil Delayed Alzheimer’s Disease-Like Symptoms by SKN-1 Pathway in C. elegans. J. Agric. Food Chem. 2017, 65, 8855–8865. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science 1990, 249, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; de Castro, E.; Hegi de Castro, S.; Cypser, J.; Henderson, S.; Tedesco, P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp. Gerontol. 2001, 36, 1609–1617. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, N.; Hughes, B.; Bigras, E.; Shoubridge, E.; Hekimi, S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: Loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005, 19, 2424–2434. [Google Scholar] [CrossRef] [Green Version]
- Melendez, A.; Talloczy, Z.; Seaman, M.; Eskelinen, E.L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008, 4, e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdelyi, P.; Takacs-Vellai, K.; Orosz, L.; Kovacs, A.L.; Csikos, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, S.-K. Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans. Antioxidants 2022, 11, 1996. https://doi.org/10.3390/antiox11101996
Park S, Park S-K. Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans. Antioxidants. 2022; 11(10):1996. https://doi.org/10.3390/antiox11101996
Chicago/Turabian StylePark, Suhyeon, and Sang-Kyu Park. 2022. "Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans" Antioxidants 11, no. 10: 1996. https://doi.org/10.3390/antiox11101996
APA StylePark, S., & Park, S.-K. (2022). Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans. Antioxidants, 11(10), 1996. https://doi.org/10.3390/antiox11101996