Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Estimation of Total Phenolics
2.3. In Vitro Antioxidant Activity
2.4. NMR Analysis
2.5. MALDI-TOF/MS Analysis
2.6. Fluorescence Quenching Assay
2.6.1. Fluorescence Spectra
2.6.2. Data Processing
2.7. UV-Vis Spectra Study
3. Results and Discussion
3.1. Quantification of the Total Phenolic Content
3.2. Antioxidant Activities of the PCT
3.3. NMR Analysis
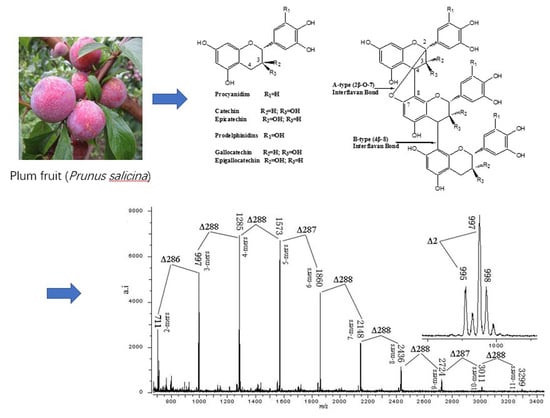
3.4. MALDI-TOF/MS Analyses
3.5. Quenching of the PCT Fluorescence Spectrum by Metal Ions
3.6. Protein-Precipitating Capacity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Choi, I.H.; Kim, D.H.; Amanullah, S.M.; Kim, S.C. Nutritional characterization of tannin rich chestnut (Castanea) and its meal for pig. J. Appl. Anim. Res. 2016, 44, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; Freitas, V.D.; Soares, S. The role of wine polysaccharides on salivary protein-tannin interaction: A molecular approach. Carbohydr. Polym. 2018, 177, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abu Zarin, M.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Du, Z.; Sheng, Z.; Jiang, W. Characterization of the interactions between banana condensed tannins and biologically important metal ions (Cu2+, Zn2+ and Fe2+). Food Res. Int. 2019, 123, 518–528. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: A meta-analysis. Biol. Trace Elem. Res. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Gujar, V.; Pundge, V.; Ottoor, D. Interaction of antihypertensive drug amiloride with metal ions in micellar medium using fluorescence spectroscopy. J. Lumin. 2015, 161, 87–94. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Gonzalez, J.M.; Halvorson, J.J.; Hagerman, A.E. Metal mobilization in soil by two structurally defined polyphenols. Chemosphere 2013, 90, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, M.A.; Frazier, R.A.; Mueller-Harvey, I.; Clifton, L.A.; Gea, A.; Green, R.J. Binding of pentagalloyl glucose to two globular proteins occurs via multiple surface sites. Biomacromolecules 2011, 12, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.P.; Green, R.J. Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): Analysis by isothermal titration calorimetry (ITC). J. Agric. Food Chem. 2015, 63, 10647–10654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hagerman, A.E. Interactions between plasma proteins and naturally occurring polyphenols. Curr. Drug Metab. 2013, 14, 432–445. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Gu, L.; Wang, L.; Qian, H.; Qi, X. Mitigation effects of proanthocyanidins with different structures on acrylamide formation in chemical and fried potato crisp models. Food Chem. 2018, 250, 98–104. [Google Scholar] [CrossRef]
- Song, W.; Zhu, X.F.; Ding, X.D.; Yang, H.B.; Qin, S.T.; Chen, H.; Wei, S.D. Structural features, antioxidant and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars. Int. J. Food Prop. 2017, 20, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Basanta, M.F.; Rizzo, S.A.; Szerman, N.; Vaudagna, S.R.; Descalzo, A.M.; Gerschenson, L.N.; Perez, C.D.; Rojas, A.M. Plum (Prunus salicina) peel and pulp microparticles as natural antioxidant additives in breast chicken patties. Food Res. Int. 2018, 106, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Taylor, A.W.; Barofsky, E.; Kennedy, J.A.; Deinzer, M.L. Hop (Humulus lupulus L.) Proanthocyanidins characterized by mass spectrometry, acid catalysis, and gel permeation chromatography. J. Agric. Food Chem. 2003, 51, 4101–4110. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Price, M.L.; Butler, L.G. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agric. Food Chem. 1977, 25, 1268–1273. [Google Scholar] [CrossRef]
- Ruan, Z.P.; Zhang, L.L.; Lin, Y.M. Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules 2008, 13, 2545–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.L.; Lin, Y.M. Antioxidant tannins from Syzygium cumini fruit. Afr. J. Biotechnol. 2009, 8, 2301–2309. [Google Scholar]
- Zhang, L.L.; Lin, Y.M. HPLC, NMR and MALDI-TOF MS analysis of condensed tannins from Lithocarpus glaber leaves with potent free radical scavenging activity. Molecules 2008, 13, 2986–2997. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.L.; Sahu, I.D.; Xu, M.; Wang, Y.M.; Hu, X.Y. Effect of metal ions on the binding reaction of (−)-epigallocatechin gallate to β-lactoglobulin. Food Chem. 2017, 221, 1923–1929. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, L.J.; Wang, J.M.; Huo, K.; Chen, C.; Zhan, W.H.; Wang, Y.L. Interaction of aconitine with bovine serum albumin and effect of atropine sulphate and glycyrrhizic acid on the binding. J. Lumin. 2012, 132, 357–361. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Miyakawa, M.E.F. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein precipitating capacity of crude canola tannins: Effect of pH, tannin, and protein concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Basanta, M.F.; Marin, A.; De Leo, S.A.; Gerschenson, L.N.; Erlejman, A.G.; Tomás-Barberán, F.A.; Rojas, A.M. Antioxidant Japanese plum (Prunus salicina) microparticles with potential for food preservation. J. Funct. Foods 2016, 24, 287–296. [Google Scholar] [CrossRef]
- Behrens, A.; Maie, N.; Knicker, H.; Kögel-Knabner, I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 2003, 62, 1159–1170. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Senter, S.D.; Forbus, W.R., Jr.; Okie, W.R. Variations in proanthocyanidins of japanese-type plums during maturation and storage. J. Sci. Food Agric. 1992, 60, 11–14. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, M.; Wang, Y.M.; Hu, X.Y. The determining of complexation reaction between Sorghum bicolor Moench proanthocyanidin and metal ions by method of fluorescence quenching. J. For. Eng. 2016, 1, 58–63. [Google Scholar]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Naczk, M.; Amarowicz, R.; Zadernowski, R.; Shahidi, F. Protein precipitating capacity of condensed tannins of beach pea, canola hulls, evening primrose and faba bean. Food Chem. 2001, 73, 467–471. [Google Scholar] [CrossRef]





| Polymer | No. of A-Type b Bonds | No. of B-Type Bonds | Calculated [M+Cs]+ | Observed [M+Cs]+ |
|---|---|---|---|---|
| Dimer | 0 | 1 | 711 | 711 |
| Trimer | 1 | 1 | 997 | 997 |
| 2 | 0 | 995 | 995 | |
| Tetramer | 1 | 2 | 1285 | 1285 |
| 2 | 1 | 1283 | 1283 | |
| Pentamer | 1 | 3 | 1573 | 1573 |
| 2 | 2 | 1571 | 1571 | |
| Hexamer | 1 | 4 | 1861 | 1860 |
| 2 | 3 | 1859 | 1858 | |
| Heptamer | 1 | 5 | 2149 | 2148 |
| 2 | 4 | 2147 | 2146 | |
| Octamer | 1 | 6 | 2437 | 2436 |
| 2 | 5 | 2435 | 2435 | |
| Nonamer | 1 | 7 | 2725 | 2724 |
| 2 | 6 | 2723 | 2722 | |
| Decamer | 2 | 7 | 3011 | 3011 |
| 3 | 6 | 3009 | 3009 | |
| Undecamer | 2 | 8 | 3299 | 3299 |
| 3 | 7 | 3297 | 3297 | |
| Dodecamer | 2 | 9 | 3587 | 3586 |
| Tridecamer | 2 | 10 | 3875 | 3875 |
| Concentration (μM) | Ka (×104 L/mol) | R | |
|---|---|---|---|
| Zn2+ a | <66.23 | 1.34 | 0.992 |
| Cu2+ a | <66.23 | 1.00 | 0.999 |
| Al3+ a | <33.22 | 7.65 | 0.999 |
| Fe2+ b | <39.84 | 1.15 | 0.985 |
| Fe3+ c | <33.22 | 6.48 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, H.; Tang, L.; Hu, X.; Xu, M. Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants 2022, 11, 714. https://doi.org/10.3390/antiox11040714
Zhang L, Zhang H, Tang L, Hu X, Xu M. Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants. 2022; 11(4):714. https://doi.org/10.3390/antiox11040714
Chicago/Turabian StyleZhang, Liangliang, He Zhang, Lihua Tang, Xinyu Hu, and Man Xu. 2022. "Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit" Antioxidants 11, no. 4: 714. https://doi.org/10.3390/antiox11040714
APA StyleZhang, L., Zhang, H., Tang, L., Hu, X., & Xu, M. (2022). Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants, 11(4), 714. https://doi.org/10.3390/antiox11040714