Protective Effects against the Development of Alzheimer’s Disease in an Animal Model through Active Immunization with Methionine-Sulfoxide Rich Protein Antigen
Abstract
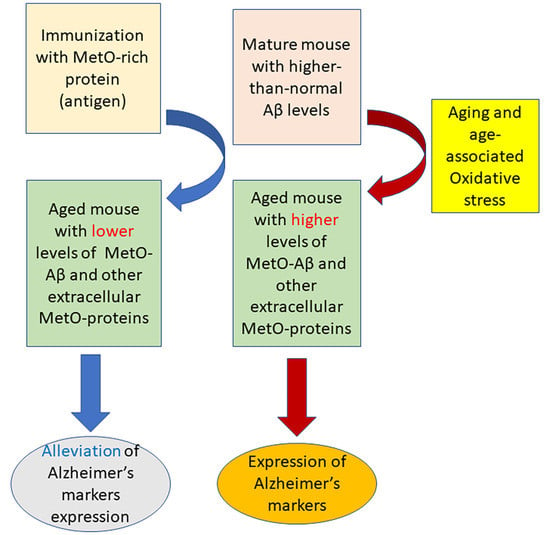
:1. Introduction
2. Material and Methods
2.1. Antibodies
2.2. MetO-Rich Protein (Antigen) Production
2.3. Mice Studies and Immunization Procedure
2.4. Mice Bio-Behavior Analyses
2.5. Y-Maze
2.6. Morris Water Maze (MWM)
2.7. Monitoring Total Aβ42 Levels in Mouse Blood-Plasma and Brain
2.8. Immunohistochemistry and Staining of Mouse Brains’ Slices
2.9. Quantification and Statistical Analyses
3. Results
3.1. Production of Anti-MetO Antibody in Rabbit and Mice
3.2. Bio-Behavioral Data
3.2.1. Y-Maze
3.2.2. Morris Water Maze (MWM)

3.3. Aβ Levels in Blood-Plasma and Brain
3.4. Levels of MetO-Proteins in Brain Cells
3.5. Indices of Gene Regulation in Brain Cells
4. Discussion
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Sultana, R.; Butterfield, D.A. Role of oxidative stress in the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid. Redox Signal. 2006, 8, 197–204. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Pappolla, M.A.; Wade, R.; Hirai, K.; Chiba, S.; Smith, M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Roychaudhuri, R.; Yang, M.; Hoshi, M.M.; Taplow, D.B. Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 2009, 284, 4749–4753. [Google Scholar] [CrossRef] [Green Version]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [CrossRef] [Green Version]
- Praticò, D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. N. Y. Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Lin, M.T.; Beal, M.F. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20 (Suppl. 2), S633–S643. [Google Scholar] [CrossRef] [Green Version]
- Bitan, G.; Tarus, B.; Vollers, S.S.; Lashuel, H.A.; Condron, M.M.; Straub, J.E.; Teplow, D.B. A molecular switch in amyloid assembly: Met35 and amyloid beta-protein oligomerization. J. Am. Chem. Soc. 2003, 25, 15359–15365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Näslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, L.; Gandy, S.E.; Winblad, B.; Greengard, P.; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.M.; Kokjohn, T.A.; Beach, T.G.; Sue, L.I.; Brune, D.; Lopez, J.C.; Kalback, W.M.; Abramowski, D.; Sturchler-Pierrat, C.; Staufenbiel, M.; et al. Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J. Biol. Chem. 2001, 276, 12991–12998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef]
- Boutte, A.M.; Woltjer, R.L.; Zimmerman, L.J.; Stamer, S.L.; Montine, K.S.; Manno, M.V.; Cimino, P.J.; Liebler, D.C.; Montine, T.J. Selectively increased oxidative modifications mapped to detergent-insoluble forms of Aβ and β-III tubulin in Alzheimer’s disease. FASEB J. 2006, 20, 1473–1483. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim. Biophys. Acta 2005, 1703, 149–156. [Google Scholar] [CrossRef]
- Triguero, L.; Singh, R.; Prabhakar, R. Comparative molecular dynamics studies of wild-type and oxidized forms of full-length Alzheimer amyloid beta-peptides Aβ(1–40) and Aβ(1–42). J. Phys. Chem. B 2008, 112, 7123–7131. [Google Scholar] [CrossRef]
- Moskovitz, J.; Maiti, P.; Lopes, D.H.; Oien, D.B.; Attar, A.; Liu, T.; Mittal, S.; Hayes, J.; Bitan, G. Induction of methionine-sulfoxide reductases protects neurons from amyloid β-protein insults in vitro and in vivo. Biochemistry 2011, 50, 10687–10697. [Google Scholar] [CrossRef] [Green Version]
- Stadtman, E.R.; Moskovitz, J.; Berlett, B.S.; Levine, R.L. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol. Cell. Biochem. 2002, 234, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Oien, D.B.; Canello, T.; Gabizon, R.; Gasset, M.; Lundquist, B.L.; Burns, J.M.; Moskovitz, J. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch. Biochem. Biophys. 2009, 485, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Qin, H.; Gao, F.P.; Cross, T.A. A systematic assessment of mature MBP in membrane protein production: Overexpression, membrane targeting and purification. Protein Expr. Purif. 2011, 80, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.; Oien, D.B.; Ersen, F.Y.; Moskovitz, J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp. Brain Res. 2007, 180, 765–774. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Minogue, A.M.; Lynch, M.A. Impaired performance of female APP/PS1 mice in the Morris water maze is coupled with increased Aβ accumulation and microglial activation. Neurodegener. Dis. 2013, 11, 33–41. [Google Scholar] [CrossRef]
- Deng, Y.; Marsh, B.M.; Moskovitz, J. Increased Levels of Protein-methionine Sulfoxide in Plasma Correlate with a Shift from a Mild Cognitive Impairment to an Alzheimer’s Disease Stage. Innov. Clin. Neurosci. 2019, 16, 29–31. [Google Scholar]
- Serneels, L.; Van Biervliet, J.; Craessaerts, K.; Dejaegere, T.; Horré, K.; Van Houtvin, T.; Esselmann, H.; Paul, S.; Schäfer, M.K.; Berezovska, O.; et al. Gamma-Secretase heterogeneity in the Aph1 subunit: Relevance for Alzheimer’s disease. Science 2009, 324, 639–642. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Ferrucci, L.; Kapogiannis, D. Safety and efficacy of active and passive immunotherapy in mild-to-moderate Alzheimer’s disease: A systematic review and network meta-analysis. Clin. Investig. Med. 2019, 42, E53–E65. [Google Scholar]
- Iwasaki, A. Immune regulation of antibody access to neuronal tissues. Trends Mol. Med. 2017, 23, 227–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewski, T.; Goñi, F. Immunotherapy for Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; Van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Aβ amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Navarro, V.; Sanchez-Mejias, E.; Jimenez, S.; Muñoz-Castro, C.; Sanchez-Varo, R.; C Davila, J.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Microglia in Alzheimer’s Disease: Activated, Dysfunctional or Degenerative. Front. Aging Neurosci. 2018, 10, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Su, J.; Sun, Y.; Feng, Y.; Shen, N.; Li, B.; Liang, Y.; Yang, X.; Wu, H.; Zhang, H.; et al. Significant upregulation of Alzheimer’s β-amyloid levels in a living system induced by extracellular elastin polypeptides. Angew. Chem. Int. Ed. 2019, 58, 18703–18709. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Ma, C.; Li, J.; Sun, Y.; Ye, F.; Liu, K.; Zhang, H. Extracellular matrix proteins involved in Alzheimer’s disease. Chemistry 2020, 26, 12101–12110. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Elastin-Derived Peptides in the Central Nervous System: Friend or Foe. Cell. Mol. Neurobiol. 2021, 2021, 1–15. [Google Scholar] [CrossRef]






| Age (Weeks) | 16 | 18 | 20 | 40 | 42 |
|---|---|---|---|---|---|
| Treatment | 1st Injection | 2nd Injection | 3rd Injection | Bio-behavioral testing | Euthanasia and post-mortem analyses on blood and brain tissues |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.S.; Gossman, K.R.; Dykstra, B.; Gao, F.P.; Moskovitz, J. Protective Effects against the Development of Alzheimer’s Disease in an Animal Model through Active Immunization with Methionine-Sulfoxide Rich Protein Antigen. Antioxidants 2022, 11, 775. https://doi.org/10.3390/antiox11040775
Smith AS, Gossman KR, Dykstra B, Gao FP, Moskovitz J. Protective Effects against the Development of Alzheimer’s Disease in an Animal Model through Active Immunization with Methionine-Sulfoxide Rich Protein Antigen. Antioxidants. 2022; 11(4):775. https://doi.org/10.3390/antiox11040775
Chicago/Turabian StyleSmith, Adam S., Kyle R. Gossman, Benjamin Dykstra, Fei Philip Gao, and Jackob Moskovitz. 2022. "Protective Effects against the Development of Alzheimer’s Disease in an Animal Model through Active Immunization with Methionine-Sulfoxide Rich Protein Antigen" Antioxidants 11, no. 4: 775. https://doi.org/10.3390/antiox11040775
APA StyleSmith, A. S., Gossman, K. R., Dykstra, B., Gao, F. P., & Moskovitz, J. (2022). Protective Effects against the Development of Alzheimer’s Disease in an Animal Model through Active Immunization with Methionine-Sulfoxide Rich Protein Antigen. Antioxidants, 11(4), 775. https://doi.org/10.3390/antiox11040775