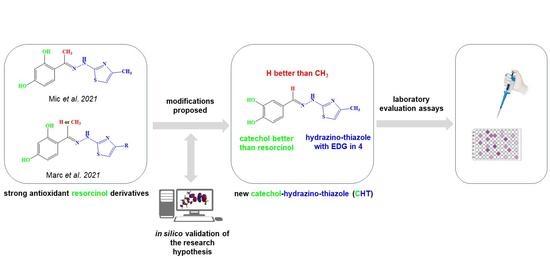
3.1. Chemical Synthesis
Compound
5 (CHT) resulted from the Hantzsch cyclization of the thiosemicarbazone of 3’,4’-dihydroxybenzaldehyde (
3). The synthesis route of CHT is presented in
Figure 1.
The intermediate compound
3 was dissolved in boiling acetone, but the final product
5 (CHT), being a hydrochloride salt, precipitates from solvent. The analysis of the spectral data (IR, MS and NMR) recorded for the intermediate compound
3 and for the final compound
5 indicates the successful obtention of the pure compounds. For the two compounds the IR and MS spectrograms are presented in
Figures S1–S4, respectively,
(E)-2-(3,4-dihydroxybenzylidene)hydrazine-1-carbothioamide (
3): yellow-beige crystalline solid; carbonization over 240 °C (lit. 245 °C [
19]); yield = 63%; FT IR (KBr) ν
max cm
−1: 3468, 3447, 3330, 3179, 1621, 1604, 1593, 1540, 1509, 1366, 1330, 1281, 1169, 1118, 842, 804, 761, 619; MS:
m/
z = 210.5 (M − 1);
(E)-2-(3,4-dihydroxybenzylidene)-1-(4-methylthiazol-2-yl)hydrazin-1-ium chloride (CHT) (5): yellow solid; carbonization over 230 °C; yield = 53%; FT IR (KBr) νmax cm−1: 3551, 3474, 3411, 3286, 3226, 3140, 3120, 2921, 1626, 1616, 1606, 1589, 1526, 1511, 1437, 1349, 1299, 1273, 1194, 1163, 1097, 965, 960, 755, 734; MS: m/z = 247.9 (M − 1); 1H-NMR (D2O, 500 MHz) δ: 2.08 (d, 3H, -CH3 (H6), J = 1.2 Hz), 6.32 (d, 1H, Th-C5-H (H5), J = 1.2 Hz), 6.71 (d, 1H, Ar (H3), J = 8.2 Hz), 9.92 (dd, 1H, Ar (H1), J = 8.2 Hz and 1.9 Hz), 7.00 (s, 1H, Ar (H2), J = 1.9 Hz), 7.71 (s, 1H, =CH- (H4)); 13C-NMR (D2O, 125 MHz) δ: 12.61 (-CH3, C11), 102.77 (Th, C9), 113.02 (Ar, C5), 115.32 (Ar, C2), 121.90 (Ar, C1), 124.88 (Ar, C6), 136.91 (Ar, C3 and C4 overlapping signals), 143.94 (Th, C8), 147.03 (Th, C10), 149.01 (=CH-, C7).
3.2. In Vitro Antioxidant, Antiradical and Chelation Assays
In order to get a complete image about the antioxidant potential of CHT, multiple assays were performed. Using in vitro methods we assessed the radical scavenging (ABTS•+, DPPH• and 1O2), the electron transfer (FRAP, RP and TAC) and, complementarily, the chelation potential of metal ions (Fe2+ and Cu2+), important due to their involvement in radical generation.
3.2.1. Antiradical Assays
The antiradical activity of CHT was determined spectrophotometrically as capacity of scavenging ABTS
•+, DPPH
• and
1O
2, compared to reference compounds ascorbic acid and/or trolox. The activity of the tested compound CHT against ABTS
•+ is presented in
Table 1. It can be seen that the activity of CHT in scavenging of ABTS
•+ is 3.16 times more intense than of trolox.
The activity of the tested compound CHT against DDPH
• is presented in
Table 2. It can be seen that CHT has a much stronger scavenging activity than the reference antioxidants used in the DDPH
• scavenging assay. CHT has a 3.28-fold lower IC
50 than trolox and a 4.94-fold lower IC
50 than ascorbic acid, respectively, by comparing the resulted IC
50.
1O
2 scavenging assay was performed by evaluation of the time-dependent degradation of DPBF molecules. The presence of
1O
2 in samples was quantified by the degradation of DPBF at room temperature by measuring UV-vis absorption spectra (
Figure 3) at the beginning, before irradiation, and after every 10 s of irradiation for a total of 80 s. No significant modifications were observed in the absorbance spectra of DPBF during or after irradiation of the control sample (
Figure 3D, gray). As expected, the biggest decrease in absorbance at 419 nm, corresponding with the decomposition of DPBF, was found in samples containing only ICG (
Figure 3C), due to singlet oxygen generation (singlet oxygen quantum yield = 0.15 [
20]). When ICG was associated with CHT or ascorbic acid as reference compound, a lower decrease in the absorbance was found at 419 nm, explained by the fact that DPBF is protected against the action of singlet oxygen radicals by the associated compounds (CHT or ascorbic acid). Comparing the magnitude of absorbance decrease in the absorbance spectra of DPBF when using the CHT + ICG vs. ascorbic acid + ICG, it can be seen that CHT better protects DPBF from radical degradation than ascorbic acid (
Figure 3A vs.
Figure 3B). Singlet oxygen quantum yield for the two samples CHT + ICG vs. ascorbic acid + ICG was found to be 0.11 vs. 0.13, respectively.
3.2.2. Electron Transfer Assays
Several assays have been applied to evaluate the potency of CHT to donating electrons, as each of these experiments uses different experimental conditions, providing a clearer picture of the activity of the evaluated compound: FRAP, RP and TAC. FRAP and RP are based on the reduction of Fe3+ to Fe2+—the first assay in acidic pH (3.6), at room temperature and in presence of 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) as ligand for the resulted ferrous ions, is given a strong blue complex, whereas RP is performed in phosphate buffer (0.2 M, pH = 6.6) at 50 °C, resulting in Perl’s Prussian blue. In the FRAP assay, the oxidant Fe3+ is found free in solution, whereas in the RP assay, it is found complexed with six cyanide ions. The TAC assay is based on the reduction of Mo6+ to Mo5+, resulting in the formation of a green complex with phosphate ions.
The results of the antioxidant activity evaluation of CHT in terms of donating electrons are presented in
Table 3.
The capacity of reduction of ferrous ions to ferric ions by CHT is significant, compared to ascorbic acid and trolox. In this context, analyzing numerically the data resulting from the FRAP and RP assays, shows the importance of performing as many antioxidant tests as possible in the evaluation of a compound in order to obtain information about its activity. In the case of the FRAP assay, at equimolecular concentrations CHT has a double activity compared to ascorbic acid and 1.71 times higher than that of trolox, in the case of the RP test the order is reversed—CHT having an activity 1.38 times higher than ascorbic acid and 2.53 times higher than trolox. This inversion of the order of activity between the two reference compounds can very well be explained due to the large differences in the experimental conditions under which the two assays are performed, as presented above: pH, temperature and ligands bound to the ferric oxidant. However, both the FRAP and RP assays indicated a strong activity in the reduction of the ferric ions by CHT, higher than ascorbic acid and trolox.
Analyzing the results of the Phosphomolybdate Assay for Total Antioxidant Capacity (TAC), CHT exhibited a similar activity to ascorbic acid and 80.10% of the activity of trolox.
3.2.3. Metal Ions Chelation Assays
Using the ferrozine method, the chelation capacity of ferrous ions of CHT was evaluated in comparison with EDTA-Na
2. The decrease in absorbance of samples in the presence of a complexant indicated that a lower quantity of ferrous ions were free in solution and a lower quantity of ferrozine–ferrous ions complexes resulted. The results of the ferrous ions chelation assay are presented in
Table 4. Although CHT has a catechol structure, the chelating activity of ferrous ions is insignificant.
CHT exhibited a significant potential in chelation of cupric ions, as presented in
Table 5. In the interval of concentrations tested, CHT presented between 81.12% of activity of EDTA-Na2 at the lowest concentration (66.66 µM) and 70.12% of activity of EDTA-Na
2 at the highest concentration (199.98 µM). The result is not surprising, because the hydrazinyl-thiazoles are known for their complexation properties of some transitional metals [
28,
29,
30,
31].
3.3. Thermodynamics of Binding Interaction
In order to get thermodynamic binding parameters (K
a, n, ΔH and ΔS), we performed ITC experiments.
Figure 4 shows the calorimetric titration profile upon the binding of CHT to protein.
Table 6 presents the thermodynamic parameters obtained by fitting experimental data with an independent binding model.
Attention was focused on the acting forces to interpret the binding mode (
Figure 4 and
Table 6). Analysis of thermodynamic data indicates a negative heat change which means the binding is exothermic with an affinity constant K
a = 7.153 × 10
3 M
−1. The binding stoichiometry of the process, n, was found to be 1.156.
The negative value of the Gibbs free energy (ΔG = −5.25 kcal/mol) suggest that the binding is a spontaneous process. The binding process had a favorable enthalpy (ΔH = 18.6 kcal/mol). The Gibbs energy value is associated with non-covalent interactions, protein–ligand and hydration effects as well as conformational changes which occur during this interaction.
The entropic effect of the binding process is ΔS = −44.8 cal/molK and brings an additional contribution to the negative Gibbs free energy. The values of ΔH and ΔS are informative for the nature of the forces governing the binding process. Both ΔH and ΔS are negative and the absolute value of ΔH highly exceeded the entropy terms TΔS.
Therefore, it suggests that hydrogen bonds or van der Waals interaction are involved in the binding mechanism. The thermodynamic signature of CHT and HSA interaction indicated that the binding is an enthalpy-driven process.
3.5. Molecular Docking
HSA, the most abundant protein in blood plasma, is responsible for the transport of fatty acids and a wide variety of pharmaceuticals and active metabolites, endo- and exogenous compounds. Albumin, a monomeric protein, has a predominantly α-helical secondary content and has a heart-shaped tertiary conformation.
As we also detailed in a previous study [
8], the molecular structure of albumin contains three homologous domains (I-III) connected with random coils, each of them divided into two subdomains (A and B) [
34]. Ghuman et al., when analyzing the drug-binding specificity of HSA, described very well the seven identified binding sites named as FA1 through FA7 where fatty acids bind to albumin [
35]. Even before determining the crystallographic structure of HSA, Sudlow’s sites were proposed [
36,
37,
38]. Sudlow’s drug site 1, DS1, is formed by an extension and widening of site FA7 towards the site FA2 and can consequently accommodate ligands bulkier than fatty acids. In a similar way, Sudlow’s drug site 2, DS2, is formed by a rearranging and merging of sites FA3 and FA4.
Albumin can then bind several types of molecules of different sizes, and even the same compound competitively, in different sites due to its intrinsic structural flexibility and many available binding sites.
Figure 12 represents the highest binding energy conformations of CHT to HSA. It binds in the FA1 pocket of the albumin and has a value of binding energy equal to −5.98 kcal/mol.
Figure 12a represents a closer look at this conformation binding site, and
Figure 12b,c represents two other conformations binding in the same pocket, FA1, and which, as we will see on the following, promote this pocket to be the most favorable one to bind CHT. To better understand the analysis procedure, after the 2000 docking runs ended, the resulting conformations were organized in clusters according to their similarity.
Figure 13 represents the histogram of the binding energy distribution of these docking conformations organized in clusters. The position of each blue rectangle in the histogram is determined by the value of the highest binding energy within each cluster and the intensity corresponds to the cluster number of members.
Analyzing this distribution, we initially observe that the cluster corresponding to the structure with the highest binding energy, represented in
Figure 12a, has 121 members. In the histogram, we can see that this is the cluster with the second highest number of members. Additionally, the other two lower binding energy clusters, which have the most numerous amounts of members, indicated with red arrows in
Figure 12, also bind in the same pocket FA1. The second highest binding energy conformation cluster has 85 members and the optimal structure has an energy of −5.89 kcal/mol, whereas the third, which is the most numerous cluster with 133 members, has the optimal structure with a binding energy of −5.48 kcal/mol. In
Figure 12b,c, mainly with comparative purpose, we represented their highest binding energy conformations. In our opinion, this particular preference is a good indication that FA1 is the most favorable pocket to bind CHT. With caution, we mention here, that the other clusters within the binding energy interval [−5.98, −5.48] kcal/mol can indicate another competitively preferred binding sites. Some of these conformations bind to the FA2 and FA5 pockets but their corresponding clusters have a lower number of members and the binding energy is lower. We mentioned this particularly because, in our docking analysis, we kept rigid the guest, HSA protein, to its crystallographically determined molecular structure and the experimental presence of water molecules and possibly ions is not taken into account in the docking procedure, but all these influences can have an impact on the effective binding conformation between CHT and HSA.
With these in mind, on the following, we focused our attention on the conformation having the highest binding energy in the FA1 pocket of albumin.
Figure 14 represents the close interaction of CHT with the protein amino acids in the FA1 pocket.
It can be seen that CHT is stabilized in the FA1 binding site principally through hydrogen bonds of both hydroxyls of catechol with phenylalanine PHE134 and tyrosine TYR161, and their benzene rings additionally define the pocket as their Pi electrons also interactwith thiazole sulfur atoms and with the catechol ring. The binding pocket is also defined further, on the catechol side of the molecule, through the interactions with LYS137, TYR138 and with GLU141 (not shown in
Figure 14a). The carboxyl of GLU141 amino acid is at about 4.5 Å from CHT H1, H2 and H4 which proved to have the most pronounced variation in selective spin-lattice relaxation in the 1H-NMR measurements. We can ascertain this interaction in
Figure 15 where we also included the receptor hydrogen-bond surface map.
The conformation represented in
Figure 14 and
Figure 15 is the one with the highest binding energy obtained after 2000 AutoDock runs and the clustering indicated clearly, in our opinion, the FA1 pocket to be the most favorable one to bind CHT. Even if we are confident that this structure has a good chance of being the effective one, we understand that the particular conformation in liquid state, which we investigated experimentally, is probably mainly favored as a result of the interaction also with the solvent molecules. Additionally, the protein is flexible and surely there will be a small adjustment of the conformation of the amino acids in the binding pocket following the interaction with CHT.