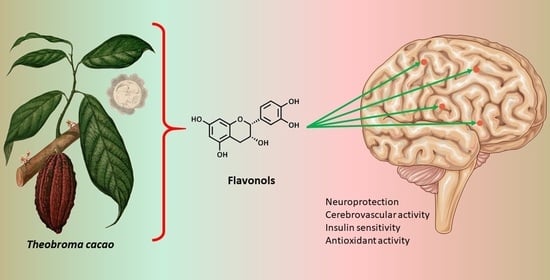
Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing
Abstract
:1. Introduction
“At no other time has Nature concentrated such a wealth of valuable nourishment into such a small space as in the cocoa bean”—(Alexander Von Humboldt).
2. Methods
3. Results and Discussion
3.1. Observational Studies
3.2. Interventional Studies
3.3. Study Quality
4. Proposed Mechanisms of Action
4.1. Neurotrophins
4.2. Cerebrovascular Action
4.3. Insulin Resistance
4.4. Gut Microbiota
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.H.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.E.; Schroeter, H.; Kuhnle, G.G.C. Assessment of the Dietary Intake of Total Flavan-3-Ols, Monomeric Flavan-3-Ols, Proanthocyanidins and Theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, S.A.; Hammerstone, J.F.; Schmitz, H.H. Chocolate Contains Additional Flavonoids Not Found in Tea. Lancet 1999, 354, 1825. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A New Process to Develop a Cocoa Powder with Higher Flavonoid Monomer Content and Enhanced Bioavailability in Healthy Humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Septianti, E.; Langkong, J. Profile of Bioactive Compounds, Antioxidant and Aromatic Component from Several Clones of Cocoa Beans during Fermentation. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 012009. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma cacao: Changes in Composition during the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Niemenak, N.; Rohsius, C.; Elwers, S.; Omokolo Ndoumou, D.; Lieberei, R. Comparative Study of Different Cocoa (Theobroma cacao L.) Clones in Terms of Their Phenolics and Anthocyanins Contents. J. Food Compos. Anal. 2006, 19, 612–619. [Google Scholar] [CrossRef]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.L.; Williamson, G. Cocoa and Health: A Decade of Research. Br. J. Nutr. 2008, 99, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; De Santis, S.; Crozier, A. Plasma Antioxidants from Chocolate. Nature 2003, 424, 1013. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3; U.S. Department of Agriculture: Washington, DC, USA, 2008; p. 173. [Google Scholar]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa Reduces Blood Pressure and Insulin Resistance and Improves Endothelium-Dependent Vasodilation in Hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The Effect of Flavanol-Rich Cocoa on the FMRI Response to a Cognitive Task in Healthy Young People. J. Cardiovasc. Pharm. 2006, 47 (Suppl. 2), S215–S220. [Google Scholar] [CrossRef]
- Sorond, F.A.; Schnyer, D.M.; Serrador, J.M.; Milberg, W.P.; Lipsitz, L.A. Cerebral Blood Flow Regulation during Cognitive Tasks: Effects of Healthy Aging. Cortex 2008, 44, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, J. Mechanisms and Evidence for the Role of Nutrition in Cognitive Ageing. Ageing Int. 2004, 29, 28–45. [Google Scholar] [CrossRef]
- Shah, H.; Albanese, E.; Duggan, C.; Rudan, I.; Langa, K.M.; Carrillo, M.C.; Chan, K.Y.; Joanette, Y.; Prince, M.; Rossor, M.; et al. Research Priorities to Reduce the Global Burden of Dementia by 2025. Lancet Neurol. 2016, 15, 1285–1294. [Google Scholar] [CrossRef]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s Disease and Other Dementias: A Priority for European Science and Society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.J. Brain Insulin Resistance and the Therapeutic Value of Insulin and Insulin-Sensitizing Drugs in Alzheimer’s Disease Neuropathology. Acta Neurol. Belg. 2022, 1–8. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain Insulin Resistance in Alzheimer’s Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Morris, M.C. Diet and Alzheimer’s Disease: What the Evidence Shows. Med. Gen. Med. 2004, 6, 48. [Google Scholar]
- Leshner, A.I.; Landis, S.; Albert, M. Preventing Cognitive Decline and Dementia: A Way Forward; National Academies Press (US): Washington, DC, USA, 2017; ISBN 978-0-309-45959-4. [Google Scholar]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Ageing, Age-Related Cardiovascular Risk and the Beneficial Role of Natural Components Intake. Int. J. Mol. Sci. 2021, 23, 183. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Majdi, A.; Mahmoudi, J.; Golzari, S.E.J.; Talebi, M. Astrocytic and Microglial Nicotinic Acetylcholine Receptors: An Overlooked Issue in Alzheimer’s Disease. J. Neural. Transm. (Vienna) 2016, 123, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological Effects of Protocatechuic Acid and Its Therapeutic Potential in Neurodegenerative Diseases: Review on the Basis of in Vitro and in Vivo Studies in Rodents and Humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Noé, V.; Peñuelas, S.; Lamuela-Raventós, R.M.; Permanyer, J.; Ciudad, C.J.; Izquierdo-Pulido, M. Epicatechin and a Cocoa Polyphenolic Extract Modulate Gene Expression in Human Caco-2 Cells. J. Nutr. 2004, 134, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. The Interactions of Flavonoids within Neuronal Signalling Pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmijn, S.; Feskens, E.J.; Launer, L.J.; Kromhout, D. Polyunsaturated Fatty Acids, Antioxidants, and Cognitive Function in Very Old Men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of Flavonoids and Risk of Dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid Intake and Cognitive Decline over a 10-Year Period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Mottaghi, T.; Amirabdollahian, F.; Haghighatdoost, F. Fruit and Vegetable Intake and Cognitive Impairment: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Clin. Nutr. 2018, 72, 1336–1344. [Google Scholar] [CrossRef]
- Scholey, A.; Owen, L. Effects of Chocolate on Cognitive Function and Mood: A Systematic Review. Nutr. Rev. 2013, 71, 665–681. [Google Scholar] [CrossRef]
- Field, D.T.; Williams, C.M.; Butler, L.T. Consumption of Cocoa Flavanols Results in an Acute Improvement in Visual and Cognitive Functions. Physiol. Behav. 2011, 103, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The Acute and Sub-Chronic Effects of Cocoa Flavanols on Mood, Cognitive and Cardiovascular Health in Young Healthy Adults: A Randomized, Controlled Trial. Front. Pharm. 2015, 6, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camfield, D.A.; Scholey, A.; Pipingas, A.; Silberstein, R.; Kras, M.; Nolidin, K.; Wesnes, K.; Pase, M.; Stough, C. Steady State Visually Evoked Potential (SSVEP) Topography Changes Associated with Cocoa Flavanol Consumption. Physiol. Behav. 2012, 105, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of Flavonoid-Rich Wine, Tea, and Chocolate by Elderly Men and Women Is Associated with Better Cognitive Test Performance. J. Nutr. 2009, 139, 120–127. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate Intake Is Associated with Better Cognitive Function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Diógenes, M.J.; de Mendonça, A.; Lunet, N.; Barros, H. Chocolate Consumption Is Associated with a Lower Risk of Cognitive Decline. J. Alzheimers Dis. 2016, 53, 85–93. [Google Scholar] [CrossRef]
- Filippini, T.; Adani, G.; Malavolti, M.; Garuti, C.; Cilloni, S.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Costa, M.; et al. Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study. Nutrients 2020, 12, 3682. [Google Scholar] [CrossRef]
- Zhong, G.-C.; Hu, T.-Y.; Yang, P.-F.; Peng, Y.; Wu, J.-J.; Sun, W.-P.; Cheng, L.; Wang, C.-R. Chocolate Consumption and All-Cause and Cause-Specific Mortality in a US Population: A Post Hoc Analysis of the PLCO Cancer Screening Trial. Aging (Albany NY) 2021, 13, 18564–18585. [Google Scholar] [CrossRef]
- Low, D.Y.; Lefèvre-Arbogast, S.; González-Domínguez, R.; Urpi-Sarda, M.; Micheau, P.; Petera, M.; Centeno, D.; Durand, S.; Pujos-Guillot, E.; Korosi, A.; et al. Diet-Related Metabolites Associated with Cognitive Decline Revealed by Untargeted Metabolomics in a Prospective Cohort. Mol. Nutr. Food Res. 2019, 63, e1900177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Domínguez, R.; Castellano-Escuder, P.; Carmona, F.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Ruigrok, S.R.; Manach, C.; Urpi-Sarda, M.; Korosi, A.; et al. Food and Microbiota Metabolites Associate with Cognitive Decline in Older Subjects: A 12-Year Prospective Study. Mol. Nutr. Food Res. 2021, 65, e2100606. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, Procyanidins, and Phenolic Microbial Metabolites after Cocoa Intake in Humans and Rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered Bile Acid Profile Associates with Cognitive Impairment in Alzheimer’s Disease-An Emerging Role for Gut Microbiome. Alzheimers Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma Phospholipids Identify Antecedent Memory Impairment in Older Adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Haller, S.; Montandon, M.-L.; Rodriguez, C.; Herrmann, F.R.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, R.S.; De Cola, M.C.; Gervasi, G.; Portaro, S.; Naro, A.; Accorinti, M.; Manuli, A.; Marra, A.; De Luca, R.; Bramanti, P. The Efficacy of Cocoa Polyphenols in the Treatment of Mild Cognitive Impairment: A Retrospective Study. Medicina 2019, 55, 156. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The Stereochemical Configuration of Flavanols Influences the Level and Metabolism of Flavanols in Humans and Their Biological Activity In Vivo. Free Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef]
- Viskupicova, J.; Ondrejovic, M.; Sturdik, E. Bioavailability and Metabolism of Flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment: The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorond, F.A.; Hurwitz, S.; Salat, D.H.; Greve, D.N.; Fisher, N.D.L. Neurovascular Coupling, Cerebral White Matter Integrity, and Response to Cocoa in Older People. Neurology 2013, 81, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P.E. High-Flavonoid Intake Induces Cognitive Improvements Linked to Changes in Serum Brain-Derived Neurotrophic Factor: Two Randomised, Controlled Trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D.L. Cerebral Blood Flow Response to Flavanol-Rich Cocoa in Healthy Elderly Humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Crews, W.D.; Harrison, D.W.; Wright, J.W. A Double-Blind, Placebo-Controlled, Randomized Trial of the Effects of Dark Chocolate and Cocoa on Variables Associated with Neuropsychological Functioning and Cardiovascular Health: Clinical Findings from a Sample of Healthy, Cognitively Intact Older Adults. Am. J. Clin. Nutr. 2008, 87, 872–880. [Google Scholar] [CrossRef] [Green Version]
- García-Cordero, J.; Pino, A.; Cuevas, C.; Puertas-Martín, V.; San Román, R.; de Pascual-Teresa, S. Neurocognitive Effects of Cocoa and Red-Berries Consumption in Healthy Adults. Nutrients 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing Dentate Gyrus Function with Dietary Flavanols Improves Cognition in Older Adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C. The Overlap between Neurodegenerative and Vascular Factors in the Pathogenesis of Dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Fisher, N.D.L.; Hollenberg, N.K. Aging and Vascular Responses to Flavanol-Rich Cocoa. J. Hypertens. 2006, 24, 1575–1580. [Google Scholar] [CrossRef] [Green Version]
- Fisher, N.D.L.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-Rich Cocoa Induces Nitric-Oxide-Dependent Vasodilation in Healthy Humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Sorond, F.A.; Kiely, D.K.; Galica, A.; Moscufo, N.; Serrador, J.M.; Iloputaife, I.; Egorova, S.; Dell’Oglio, E.; Meier, D.S.; Newton, E.; et al. Neurovascular Coupling Is Impaired in Slow Walkers: The MOBILIZE Boston Study. Ann. Neurol. 2011, 70, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suominen, M.H.; Laaksonen, M.M.L.; Salmenius-Suominen, H.; Kautiainen, H.; Hongisto, S.-M.; Tuukkanen, K.; Jyväkorpi, S.K.; Pitkälä, K.H. The Short-Term Effect of Dark Chocolate Flavanols on Cognition in Older Adults: A Randomized Controlled Trial (FlaSeCo). Exp. Gerontol. 2020, 136, 110933. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.P.; Wall, M.; Yeung, L.-K.; Feng, T.; Feng, X.; Provenzano, F.; Schroeter, H.; Lauriola, V.; Brickman, A.M.; Small, S.A. Insights into the Role of Diet and Dietary Flavanols in Cognitive Aging: Results of a Randomized Controlled Trial. Sci. Rep. 2021, 11, 3837. [Google Scholar] [CrossRef] [PubMed]
- Halib, H.; Ismail, A.; Mohd Yusof, B.-N.; Osakabe, N.; Mat Daud, Z.A. Effects of Cocoa Polyphenols and Dark Chocolate on Obese Adults: A Scoping Review. Nutrients 2020, 12, 3695. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Combet, E.; Pinto, P.; Mena, P.; Dall’Asta, M.; Garcia-Aloy, M.; Rodríguez-Mateos, A.; Gibney, E.R.; Dumont, J.; Massaro, M.; et al. A Systematic Review and Meta-Analysis of the Effects of Flavanol-Containing Tea, Cocoa and Apple Products on Body Composition and Blood Lipids: Exploring the Factors Responsible for Variability in Their Efficacy. Nutrients 2017, 9, 746. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool (accessed on 22 May 2022).
- Bors, W.; Michel, C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.-O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the Putative Catechin and Epicatechin Transport across Blood-Brain Barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef]
- Nehlig, A. The Neuroprotective Effects of Cocoa Flavanol and Its Influence on Cognitive Performance. Br. J. Clin. Pharm. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin Mediates Beneficial Effects of Flavanol-Rich Cocoa on Vascular Function in Humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenberg, N.K.; Fisher, N.D.L.; McCullough, M.L. Flavanols, the Kuna, Cocoa Consumption, and Nitric Oxide. J. Am. Soc. Hypertens. 2009, 3, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular Effects of Cocoa Rich in Flavan-3-Ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef]
- Schewe, T.; Steffen, Y.; Sies, H. How Do Dietary Flavanols Improve Vascular Function? A Position Paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef]
- Kerimi, A.; Williamson, G. The Cardiovascular Benefits of Dark Chocolate. Vasc. Pharm. 2015, 71, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Owen, L.; Sunram-Lea, S.I. Metabolic Agents That Enhance ATP Can Improve Cognitive Functioning: A Review of the Evidence for Glucose, Oxygen, Pyruvate, Creatine, and L-Carnitine. Nutrients 2011, 3, 735–755. [Google Scholar] [CrossRef] [Green Version]
- Nagahama, Y.; Nabatame, H.; Okina, T.; Yamauchi, H.; Narita, M.; Fujimoto, N.; Murakami, M.; Fukuyama, H.; Matsuda, M. Cerebral Correlates of the Progression Rate of the Cognitive Decline in Probable Alzheimer’s Disease. Eur. Neurol. 2003, 50, 1–9. [Google Scholar] [CrossRef]
- Heiss, C.; Finis, D.; Kleinbongard, P.; Hoffmann, A.; Rassaf, T.; Kelm, M.; Sies, H. Sustained Increase in Flow-Mediated Dilation after Daily Intake of High-Flavanol Cocoa Drink over 1 Week. J. Cardiovasc. Pharm. 2007, 49, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The Effect of Flavanol-Rich Cocoa on Cerebral Perfusion in Healthy Older Adults during Conscious Resting State: A Placebo Controlled, Crossover, Acute Trial. Psychopharmacology (Berl) 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of Chocolate, Cocoa, and Flavan-3-Ols on Cardiovascular Health: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Lapre, E.; Laksir, H.; Puget, E. Insulin Resistance, Diabetes and Cognitive Function: Consequences for Preventative Strategies. Diabetes Metab. 2010, 36, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.F.; Rojo, L.; Navarrete, L.P.; Farías, G.; Reyes, P.; Maccioni, R.B. Insulin Resistance and Alzheimer’s Disease: Molecular Links & Clinical Implications. Curr. Alzheimer Res. 2008, 5, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, T.M.; Craft, S. The Role of Insulin in the Vascular Contributions to Age-Related Dementia. Biochim. Biophys. Acta 2016, 1862, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Yavuz, S. Metabolic Actions of Angiotensin II and Insulin: A Microvascular Endothelial Balancing Act. Mol. Cell. Endocrinol. 2013, 378, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of Angiotensin Converting Enzyme (ACE) Activity by Flavan-3-Ols and Procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Schroeter, H.; Balzer, J.; Kleinbongard, P.; Matern, S.; Sies, H.; Kelm, M. Endothelial Function, Nitric Oxide, and Cocoa Flavanols. J. Cardiovasc. Pharm. 2006, 47 (Suppl. 2), S128–S135. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the Intestinal Glucose Transporter GLUT2 by Flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [Green Version]
- de la Monte, S.M. Insulin Resistance and Alzheimer’s Disease. BMB Rep. 2009, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hölscher, C. Common Pathological Processes in Alzheimer Disease and Type 2 Diabetes: A Review. Brain Res. Rev. 2007, 56, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorius, T.; Peter, A.; Heni, M.; Maetzler, W.; Fritsche, A.; Häring, H.-U.; Hennige, A.M. The Brain Response to Peripheral Insulin Declines with Age: A Contribution of the Blood-Brain Barrier? PLoS ONE 2015, 10, e0126804. [Google Scholar] [CrossRef] [Green Version]
- de la Monte, S.M.; Tong, M.; Daiello, L.A.; Ott, B.R. Early-Stage Alzheimer’s Disease Is Associated with Simultaneous Systemic and Central Nervous System Dysregulation of Insulin-Linked Metabolic Pathways. J. Alzheimers Dis. 2019, 68, 657–668. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment: A Pilot Clinical Trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Reger, M.A.; Watson, G.S.; Frey, W.H.; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of Intranasal Insulin on Cognition in Memory-Impaired Older Adults: Modulation by APOE Genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Mayeux, R. Dietary Factors and Alzheimer’s Disease. Lancet Neurol. 2004, 3, 579–587. [Google Scholar] [CrossRef]
- Zhu, F. Proanthocyanidins in Cereals and Pseudocereals. Crit. Rev. Food Sci. Nutr. 2019, 59, 1521–1533. [Google Scholar] [CrossRef]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in Human Plasma: In Vivo Determination and Effect of Chocolate Consumption on Plasma Oxidation Status. J. Nutr. 2000, 130, 2109S–2114S. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.-W.; Ryu, S.-N.; Kim, D.-H. Anti-Inflammatory Effects of Black Rice, Cyanidin-3-O-Beta-D-Glycoside, and Its Metabolites, Cyanidin and Protocatechuic Acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The Bioactivity of Dietary Anthocyanins Is Likely to Be Mediated by Their Degradation Products. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- SMART. Available online: https://smart.servier.com/ (accessed on 26 July 2021).
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué I Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Detection of Alzheimer’s Disease and Other Dementias in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2015, 3, CD010783. [Google Scholar] [CrossRef]



| Reference | Type of Study | n | Participants | Age | Duration | Outcomes | Main Findings |
|---|---|---|---|---|---|---|---|
| Nurk et al., 2009 [38] | Cross-sectional | 2031 | Healthy | 70–74 | / | Association with neuropsychological tests | Little cognitive improvement associated with chocolate |
| Crichton et al., 2016 [39] | Cross-sectional | 968 | Community-dwelling participants | 23–98 | / | Association with neuropsychological tests | Better scores among chocolate users without association with CD |
| Moreira et al., 2016 [40] | Prospective cohort | 309 | Healthy | >65 | 4 years mean of follow-up | MMSE score | Chocolate intake was associated with a lower risk of CD (RR = 0.59, 95% CI: 0.38–0.92) |
| Filippini et al., 2020 [41] | Case–control | 54 + 54 | EOD patients with their caregivers as control | 65 as mean | / | Association between EOD and dietary factors | Lower risk of EOD with the upper level of chocolate use |
| Zhong et al., 2021 [42] | Prospective cohort | 91,891 | Patients with prostate, lung, colorectal and ovarian cancer | 55–74 | Up to 15.5 years of follow-up | Association between chocolate consumption and mortality | Strong inverse association between chocolate consumption and mortality for AD (HR = 0.69, 95% CI: 0.49–0.99) |
| Low et al., 2019 [43] | Nested Case–control | 209 + 209 | CD with matched controls | >65 | Follow-up of 12 years | Metabolomics of diet-related biomolecules and cognition using MMSE and other neuropsychological tests | Cocoa metabolites were inversely associated with CD |
| González-Domínguez et al., 2021 [44] | Nested Case–control | 209 + 209 and 212 + 212 | CD with matched controls | >65 | Follow-up of 12 years | Metabolomics of diet-related biomolecules and cognition using MMSE and other neuropsychological tests | Inverse association between 3-methylxanthine metabolite and subsequent CD |
| Haller et al., 2018 [49] | Prospective cohort | 145 | Healthy | 69–86 | Follow-up of 3 years | Neuropsychological tests and MRI | No correlation between chocolate consumption and cognition or MRI |
| Calabrò et al., 2019 [50] | Retrospective | 55 | MCI using Mexenion® | 56–75 | 1 year | MMSE | Cocoa polyphenols intake was related to slowing down the cognitive worsening |
| Reference | Type of Study | n | Participants | Age | Duration | Intervention | Outcomes | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Sorond et al., 2008 [57] | Single-arm intervention | 13 | Healthy | 59–83 | 2 weeks | 900 mg cocoa flavanols drink per day | Transcranial Doppler ultrasound | Mean blood flow velocity increased after 1 or 2 weeks of flavanols intake |
| Sorond et al., 2008 [57] | Parallel RCT | 21 | Healthy | 59–83 | 1 week | 900 mg cocoa flavanols drink per day or placebo | Transcranial Doppler ultrasound | No cerebrovascular resistance or vasoreactivity improvements after the intervention. Mean blood flow velocity response increased in the intervention group but without statistical significance among groups |
| Sorond et al., 2013 [54] | Parallel RCT | 60 | With vascular risk factors, cognitively intact | >65 | 30 days | 609 mg cocoa flavanols drink or 13 mg, two times per day | MMSE, cerebral blood flow velocity and MRI | Cocoa consumption was associated with neurovascular coupling and MMSE improvements in neurovascular coupling impaired patients |
| Desideri et al., 2012 [53] | Parallel RCT | 90 | MCI | 71 ± 5 | 8 weeks | 690, 520 or 45 mg cocoa flavanols drink per day | MMSE and TMT | Improvement in cognitive performance associated with cocoa use, also at an intermediate dosage |
| Mastroiacovo et al., 2015 [55] | Parallel RCT | 90 | Cognitively intact | 61–85 | 8 weeks | 690, 520 or 45 mg cocoa flavanols drink per day | MMSE, TMT and VFT | Improvement in cognitive performance associated with cocoa use, also at an intermediate dosage |
| Crews et al., 2008 [58] | Parallel RCT | 101 | Cognitively intact | >60 | 6 weeks | 37 g dark chocolate with 397.30 mg proanthocyanins/g and 237 mL of cocoa beverage with 357.41 mg proanthocyanins/g per day or placebo (0.2 and 40.87 mg/g, respectively) | Neuropsychological test battery | No improvement after cocoa and chocolate intake |
| Suominen et al., 2020 [65] | Parallel RCT | 100 | Cognitively intact | 65–75 | 8 weeks | 50 g dark chocolate with 410 mg or 86 mg of flavanols per day | TMT and VFT | No improvement after cocoa and chocolate intake |
| Neshatdoust et al., 2016 [56] | Cross-over RCT | 40 | Healthy | 62–75 | 12 weeks | 494 mg or 23 mg flavanols cocoa drink per day | Serum BDNF levels, neuropsychological test battery | Significant increase in BDNF levels after high flavanols intake with improved cognitive function |
| García-Cordero et al., 2021 [59] | Parallel RTC | 60 | Healthy | 50–75 | 12 weeks | Cocoa powder with 200 mg of flavanols per day, red berries mixture or both | Serum BDNF levels, neuropsychological test battery | No changes in BDNF levels. Neurocognitive enhancement in all groups |
| Brickman et al., 2014 [60] | Parallel RCT | 41 | Healthy | 50–69 | 3 months | Cocoa with 900 mg or 45 mg of flavanol per day | CBV-fMRI, ModBent Task and mod Rey auditory learning task | Correlation between increased cerebral blood volume and better performance in the dentate gyrus after the high flavanol intake |
| Sloan et al., 2021 [66] | Parallel RCT | 211 | Healthy | 50–75 | 12 weeks | 260, 510 or 770 mg of cocoa flavanol capsules per day or placebo | CBV-fMRI, ModBent Task and mod Rey auditory learning task, List-Sorting Working Memory Test | Object-recognition list-sorting tasks were not improved after the intervention. An improvement in list-learning performance was associated with the cocoa intervention |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeli, C.; Lombardo, M.; Storz, M.A.; Ottaviani, M.; Rizzo, G. Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing. Antioxidants 2022, 11, 1353. https://doi.org/10.3390/antiox11071353
Zeli C, Lombardo M, Storz MA, Ottaviani M, Rizzo G. Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing. Antioxidants. 2022; 11(7):1353. https://doi.org/10.3390/antiox11071353
Chicago/Turabian StyleZeli, Corinna, Mauro Lombardo, Maximilian Andreas Storz, Morena Ottaviani, and Gianluca Rizzo. 2022. "Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing" Antioxidants 11, no. 7: 1353. https://doi.org/10.3390/antiox11071353
APA StyleZeli, C., Lombardo, M., Storz, M. A., Ottaviani, M., & Rizzo, G. (2022). Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing. Antioxidants, 11(7), 1353. https://doi.org/10.3390/antiox11071353