Tolerance Assessment of Atractylodes macrocephala Polysaccharide in the Diet of Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
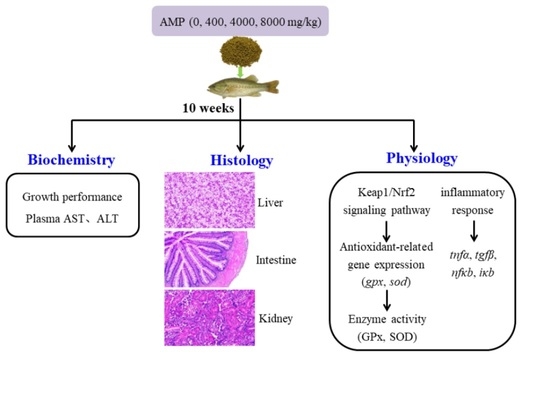
2.2. Feeding Trial and Sampling
2.3. Chemical Composition Analysis
2.4. Biochemical Analysis
2.5. Histological Analysis
2.6. RNA Extraction and Real-Time Quantitative PCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Body Composition
3.2. Plasma Metabolites
3.3. Nrf2/Keap1 Signaling Pathway and Antioxidant-Related Genes in the Liver and Intestine
3.4. Activities of Antioxidant Enzymes in the Liver
3.5. Expression of Inflammatory-Related Genes in the Liver, Intestine, and Kidney
3.6. Histological Analysis of the Liver, Intestine, and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department: Rome, Italy, 2020. [Google Scholar]
- Vazquez-Salgado, L.; Olveira, J.; Dopazo, C.; Bandin, I. Effect of rearing density on nervous necrosis virus infection in Senegalese sole (Solea senegalensis). J. Fish Dis. 2021, 44, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Khati, A.; Chauhan, R.; Arya, P.; Semwal, A. A review on feed additives used in fish diet. Int. J. Environ. Agric. Biotechnol. 2021, 6, 2. [Google Scholar] [CrossRef]
- Yin, G.; Li, W.; Lin, Q.; Lin, X.; Lin, J.; Zhu, Q.; Jiang, H.; Huang, Z. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish Shellfish. Immunol. 2014, 41, 402–406. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B.; Chen, J.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S.; Yao, M. Supplementing sulfate-based alginate polysaccharide improves pacific white shrimp (Litopenaeus vannamei) fed fishmeal replacement with cottonseed protein concentrate: Effects on growth, intestinal health, and disease resistance. Aquac. Nutr. 2022, 2022, 7132362. [Google Scholar] [CrossRef]
- Chang, Z.; Ge, Q.; Sun, M.; Wang, Q.; Lv, H.; Li, J. Immune responses by dietary supplement with Astragalus polysaccharides in the pacific white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Feng, J.; Chang, X.; Zhang, Y.; Lu, R.; Meng, X.; Song, D.; Yan, X.; Zhang, J.; Nie, G. Characterization of a polysaccharide HP-02 from honeysuckle flowers and its immunoregulatory and anti-Aeromonas hydrophila effects in Cyprinus carpio L. Int. J. Biol. Macromol. 2019, 140, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ji, H.; Dong, X.; Liu, A. An alcohol-soluble polysaccharide from Atractylodes macrocephala Koidz induces apoptosis of Eca-109 cells. Carbohydr. Polym. 2019, 226, 115136. [Google Scholar] [CrossRef]
- Feng, Y.; Ji, H.; Dong, X.; Yu, J.; Liu, A. Polysaccharide extracted from Atractylodes macrocephala Koidz (PAMK) induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2019, 137, 604–611. [Google Scholar] [CrossRef]
- Yang, L.; Yu, H.; Hou, A.; Man, W.; Wang, S.; Zhang, J.; Wang, X.; Zheng, S.; Jiang, H.; Kuang, H. A review of the ethnopharmacology, phytochemistry, pharmacology, application, quality control, processing, toxicology, and pharmacokinetics of the dried rhizome of Atractylodes macrocephala. Front. Pharmacol. 2021, 12, 727154. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Tian, Y.; Guo, S.; Huang, Y.; Xu, D.; Cao, N. Polysaccharide of Atractylodes macrocephala Koidz enhances cytokine secretion by stimulating the TLR4-MyD88-NF-κB signaling pathway in the mouse spleen. J. Med. Food 2019, 22, 937–943. [Google Scholar] [CrossRef]
- Guo, S.; Li, W.; Chen, F.; Yang, S.; Huang, Y.; Tian, Y.; Xu, D.; Cao, N. Polysaccharide of Atractylodes macrocephala Koidz regulates LPS-mediated mouse hepatitis through the TLR4-MyD88-NFκB signaling pathway. Int. Immunopharmacol. 2021, 98, 107692. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, X.; Xu, S.; Cao, N.; Li, B.; Chen, W.; Yang, B.; Yuan, M.; Xu, D. Lipopolysaccharide-induced splenic ferroptosis in goslings was alleviated by polysaccharide of Atractylodes macrocephala Koidz associated with pro-inflammatory factors. Poult. Sci. 2022, 101, 101725. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Li, Q.; Liu, J.; Hu, Y. Selenylation modification of Atractylodes macrocephala polysaccharide and evaluation of antioxidant activity. Adv. Polym. Technol. 2019, 2019, 8191385. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Xu, R.; Zhang, X.; Sun, Y.; Feng, Q.; Li, Z.; Xu, J.; Xie, Z.; Zhang, Z.; et al. Sesquiterpene lactams and lactones with antioxidant potentials from Atractylodes macrocephala discovered by molecular networking strategy. Front. Nutr. 2022, 9, 865257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Pan, S.; Wu, S. Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2020, 147, 29–33. [Google Scholar] [CrossRef]
- Pierri, B.; Silva, A.; Cadorin, D.; Ferreira, T.; Mourino, J.; Filer, K.; Pettigrew, J.; Fracalossi, D. Different levels of organic trace minerals in diets for Nile tilapia juveniles alter gut characteristics and body composition, but not growth. Aquac. Nutr. 2021, 27, 176–186. [Google Scholar] [CrossRef]
- Ye, C.; Wang, E.; He, S.; Wang, K.; Geng, Y.; He, Q.; Yang, Q.; Liu, T.; Xie, H. Subchronic toxicity and hepatocyte apoptosis of dietary olaquindox in common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2018, 164, 131–139. [Google Scholar] [CrossRef]
- Wu, Z.; Pang, S.; Chen, X.; Yu, Y.; Zhou, J.; Chen, X.; Pang, L. Effect of Coriolus versicolor polysaccharides on the hematological and biochemical parameters and protection against Aeromonas hydrophila in allogynogenetic crucian carp (Carassius auratus gibelio). Fish Physiol. Biochem. 2013, 39, 181–190. [Google Scholar] [CrossRef]
- Ren, S.; Cai, C.; Cui, G.; Ni, Q.; Jiang, R.; Su, X.; Wang, Q.; Chen, W.; Zhang, J.; Wu, P. High dosages of pectin and cellulose cause different degrees of damage to the livers and intestines of Pelteobagrus fulvidraco. Aquaculture 2020, 514, 734445. [Google Scholar] [CrossRef]
- Bureau MOAA. 2021 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Poudyal, S.; Pulpipat, T.; Wang, P.; Chen, S. Comparison of the pathogenicity of Francisella orientalis in Nile tilapia (Oreochromis niloticus), Asian seabass (Lates calcarifer) and largemouth bass (Micropterus salmoides) through experimental intraperitoneal infection. J. Fish Dis. 2020, 43, 1097–1106. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, X.; Zeng, K.; Xie, D.; Song, C.; Tam, K.; Liu, Z.; Zhou, T.; Li, W. Construction of a DNA vaccine and its protective effect on largemouth bass (Micropterus salmoides) challenged with largemouth bass virus (LMBV). Fish Shellfish Immunol. 2020, 106, 103–109. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Sun, H.; Liao, R.; Wei, Y.; Zhang, T.; Chen, Y.; Lin, S. Effects of herbal extracts (Foeniculum vulgare and Artemisia annua) on growth, liver antioxidant capacity, intestinal morphology and microorganism of juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2022, 23, 101081. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; Fang, H.; Tian, L.; Liu, Y.; Niu, J. Chlorogenic acid improves health in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets: Involvement of lipid metabolism, antioxidant ability, inflammatory response, and intestinal integrity. Aquaculture 2021, 545, 737169. [Google Scholar] [CrossRef]
- Chen, W.; Chang, K.; Chen, J.; Zhao, X.; Gao, S. Dietary sodium butyrate supplementation attenuates intestinal inflammatory response and improves gut microbiota composition in largemouth bass (Micropterus salmoides) fed with a high soybean meal diet. Fish Physiol. Biochem. 2021, 47, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- AOAC, Association of Official Analytical Chemists. Official Methods of Analysis. AOAC 2006, 16, 0066-0961X. [Google Scholar]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y. Vitamin C attenuates oxidative stress, inflammation, and apoptosis induced by acute hypoxia through the Nrf2/Keap1 signaling pathway in gibel carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Farnese, F.; Oliveira, J.; Paiva, E.; Menezes-Silva, P.; da Sliva, A.; Campos, F.; Ribeiro, C. The involvement of nitric oxide in integration of plant physiological and ultrastructural adjustments in response to arsenic. J. Front. Plant Sci. 2017, 8, 516. [Google Scholar] [CrossRef]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Xu, W.; Dong, B.; Jin, J.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Dissimilar regulation of glucose and lipid metabolism by leptin in two strains of gibel carp (Carassius gibelio). Br. J. Nutr. 2021, 125, 1215–1229. [Google Scholar] [CrossRef]
- Van Doan, H.; Tapingkae, W.; Moonmanee, T.; Seepai, A. Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Cui, L.; Xu, W.; Ai, Q.; Wang, D.; Mai, K. Effects of dietary chitosan oligosaccharide complex with rare earth on growth performance and innate immune response of turbot, Scophthalmus maximus L. Aquac. Res. 2013, 44, 683–690. [Google Scholar] [CrossRef]
- Akbary, P.; Aminikhoei, Z. Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 2018, 30, 1345–1353. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.; Zhang, J.; Fang, W.; Mai, K.; Ai, Q. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Traifalgar, R.; Kira, H.; Tung, H.; Michael, F.; Laining, A.; Yokoyama, S.; Ishikawa, M.; Koshio, S.; Serrano, A.; Corre, V. Influence of dietary fucoidan supplementation on growth and immunological response of juvenile Marsupenaeus japonicus. J. World Aquac. Soc. 2010, 41, 235–244. [Google Scholar] [CrossRef]
- Gabriel, N.; Wilhelm, M.; Habte-Tsion, H.; Chimwamurombe, P.; Omoregie, E.; Iipinge, L.; Shimooshili, K. Effect of dietary Aloe vera polysaccharides supplementation on growth performance, feed utilization, hemato-biochemical parameters, and survival at low pH in African catfish (Clarias gariepinus) fingerlings. Int. Aquat. Res. 2019, 11, 57–72. [Google Scholar] [CrossRef]
- Gabriel, N.; Qiang, J.; Ma, X.; Xu, P.; Nakwaya, D. Effects of dietary Aloe vera crude extracts on digestive enzyme activities and muscle proximate composition of GIFT tilapia juveniles. South Afr. J. Anim. Sci. 2017, 47, 904–913. [Google Scholar] [CrossRef]
- Heidarieh, M.; Mirvaghefi, A.; Sepahi, A.; Sheikhzadeh, N.; Alishahbazfar, A.; Akbari, M. Effects of dietary Aloe vera on growth performance, skin and gastrointestine morphology in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2013, 13, 367–373. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.; Das, K.; Agrawal, P.; Paital, B. Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108971. [Google Scholar] [CrossRef]
- Loro, V.; Jorge, M.; da Silva, K.; Wood, C. Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus: Protective effects of salinity. Aquat. Toxicol. 2012, 110, 187–193. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, C.; Wang, P.; Wang, S.; Lin, H.; Qiu, L. The response of glutathione peroxidase 1 and glutathione peroxidase 7 under different oxidative stresses in black tiger shrimp, Penaeus monodon. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 217, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Risha, E.; AbdelHamid, F.; Mahgoub, H.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 38, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y. The growth performance and nonspecific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary Porphyra yezoensis polysaccharide supplementation. Fish Shellfish. Immunol. 2019, 87, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, R.; Li, M.; Zhou, Q.; Liang, X.; Elmada, Z. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2014, 41, 264–270. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Yuan, Y.; Zhang, Z.; Jiang, B.; Yang, S.; Jian, J. Silencing of Nrf2 in Litopenaeus vannamei, decreased the antioxidant capacity, and increased apoptosis and autophagy. Fish Shellfish Immunol. 2022, 122, 257–267. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Y.; Deng, M.; Liu, Y.; Wang, S.; He, X.; Allaire-Leung, M.; Wan, J.; Zou, Y.; Yang, C. Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae. Chemosphere 2019, 226, 696–703. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, L.; Zhao, J.; Xu, W.; Guo, Z.; Zhang, A.; Li, M. Dietary Taraxacum mongolicum polysaccharide ameliorates the growth, immune response, and antioxidant status in association with NF-κB, Nrf2 and TOR in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2022, 547, 737522. [Google Scholar] [CrossRef]
- Mohammadi, G.; Karimi, A.; Hafezieh, M.; Dawood, M.; Abo-Al-Ela, H. Pistachio hull polysaccharide protects Nile tilapia against LPS-induced excessive inflammatory responses and oxidative stress, possibly via TLR2 and Nrf2 signaling pathways. Fish Shellfish Immunol. 2022, 121, 276–284. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Arockiaraj, J.; Jagruthi, C. Efficacy of ulvan on immune response and immuno-antioxidant gene modulation in Labeo rohita against columnaris disease. Fish Shellfish Immunol. 2021, 117, 262–273. [Google Scholar] [CrossRef]
- Brum, A.; Cardoso, L.; Chagas, E.; Chaves, F.; Mourino, J.; Martins, M. Histological changes in nile tilapia fed essential oils of clove basil and ginger after challenge with Streptococcus agalactiae. Aquac. 2018, 490, 98–107. [Google Scholar] [CrossRef]
- Kracizy, R.; Brazao, C.; Viott, A.; Ribeiro, K.; Koppenol, A.; Dos Santos, A.; Ballester, E. Evaluation of aflatoxin and fumonisin in the diet of pacific white shrimp (Litopenaeus vannamei) on their performance and health. Aquaculture 2021, 544, 737051. [Google Scholar] [CrossRef]
- Qiang, J.; Khamis, O.; Jiang, H.; Cao, Z.; He, J.; Tao, Y.; Xu, P.; Bao, J. Effects of dietary supplementation with apple peel powder on the growth, blood and liver parameters, and transcriptome of genetically improved farmed tilapia (GIFT, Oreochromis niloticus). PLoS ONE 2019, 14, e0224995. [Google Scholar] [CrossRef]
- Miandare, H.; Niknejad, M.; Shabani, A.; Safari, R. Exposure of Persian sturgeon (Acipenser persicus) to cadmium results in biochemical, histological and transcriptional alterations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pan, X.; Cheng, W.; Cheng, Y.; Yin, Y.; Chen, J.; Xu, G.; Xie, L. Serum biochemistry, histology and transcriptomic profile analysis reflect liver inflammation and damage following dietary histamine supplementation in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2018, 77, 83–90. [Google Scholar] [CrossRef]
- Molinari, G.; Wojno, M.; McCracken, V.; Kwasek, K. The use of dipeptide supplementation as a means of mitigating the negative effects of dietary soybean meal on zebrafish Danio rerio. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110958. [Google Scholar] [CrossRef]
- Qiu, H.; Veeraperumal, S.; Lv, J.; Wu, T.; Zhang, Z.; Zeng, Q.; Liu, Y.; Chen, X.; Aweya, J.; Cheong, K. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- El-Sayed, A.; Tammam, M.; Makled, S. Lecithin-containing bioemulsifier boosts growth performance, feed digestion and absorption and immune response of adult nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2021, 27, 757–770. [Google Scholar] [CrossRef]
- Mahgoub, H.; El-Adl, M.; Ghanem, H.; Martyniuk, C. The effect of fucoidan or potassium permanganate on growth performance, intestinal pathology, and antioxidant status in Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2020, 46, 2109–2131. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, S.; Balasubramanian, B.; Zeng, F.; Sun, C.; Pang, H. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2020, 104, 202–212. [Google Scholar] [CrossRef]
- Xie, S.; Zheng, L.; Wan, M.; Niu, J.; Liu, Y.; Tian, L. Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 82, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, S.; Karthika, P.; Suvenitha, K.; Kadirvelu, K.; Ramesh, M. Dose-dependent molecular responses of Labeo rohita to triphenyl phosphate. Chem. Res. Toxicol. 2021, 34, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Berntssen, M.; Betancor, M.; Caballero, M.; Hillestad, M.; Rasinger, J.; Hamre, K.; Sele, V.; Amlund, H.; Ornsrud, R. Safe limits of selenomethionine and selenite supplementation to plant-based Atlantic salmon feeds. Aquaculture 2018, 495, 617–630. [Google Scholar] [CrossRef]
- Gan, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhou, X. Erucic acid inhibits growth performance and disrupts intestinal structural integrity of on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2019, 513, 734437. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Kotas, M.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Shah, R.; Hurley, C.; Posch, P. A molecular mechanism for the differential regulation of TGF-β1 expression due to the common SNP− 509C-T (c.− 1347C> T). Hum. Genet. 2006, 120, 461–469. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 99, 603–608. [Google Scholar] [CrossRef]
- Feng, J.; Cai, Z.; Zhang, X.; Chen, Y.; Chang, X.; Wang, X.; Qin, C.; Yan, X.; Ma, X.; Zhang, J. The Effects of oral Rehmannia glutinosa polysaccharide administration on immune responses, antioxidant activity and resistance against Aeromonas hydrophila in the common carp, Cyprinus carpio L. Front. Immunol. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, H.H.; Liu, Z.Z.; Chen, J.M.; Zhang, C.W.; Gao, B.Y.; Niu, J. Responses in growth performance, enzymatic activity, immune function and liver health after dietary supplementation of Porphyridium sp. in juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 27, 679–690. [Google Scholar] [CrossRef]







| Ingredients | A0 | A400 | A4000 | A8000 |
|---|---|---|---|---|
| Fish meal a | 40 | 40 | 40 | 40 |
| Wheat gluten | 6.5 | 6.5 | 6.5 | 6.5 |
| Casein | 18.5 | 18.5 | 18.5 | 18.5 |
| Flour | 5 | 5 | 5 | 5 |
| Cassava starch | 10 | 10 | 10 | 10 |
| Fish oil | 6 | 6 | 6 | 6 |
| Vitamin & mineral premix b | 1 | 1 | 1 | 1 |
| Monocalcium phosphate | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 |
| Bentonite | 11.40 | 11.36 | 11.00 | 10.60 |
| AMP (mg/kg) c | 0 | 400 | 4000 | 8000 |
| Chemical composition | ||||
| Moisture | 7.91 | 7.97 | 7.16 | 7.28 |
| Crude protein | 52.07 | 52.14 | 51.29 | 51.70 |
| Crude lipid | 9.50 | 9.06 | 9.04 | 9.35 |
| Gene Name | Sense and Antisense Primer (5′-3′) | Accession No. | Product Length (bp) |
|---|---|---|---|
| Transforming growth factor β (tgfβ) | ACAGTGGGCAATGTAAGCGGTA | XM_038693206.1 | 232 |
| TGTCTGGTGGGCTCTCGGTCTG | |||
| Tumor necrosis factor α (tnfα) | CAAGTGTCAAACCCAGTTCCAA | XM_038723994.1 | 154 |
| ATTTGCCTCAATGTGTGACGAT | |||
| Superoxide dismutase (sod) | CAGTTACCAGTGTGTCGGCTCT | XM_038727054.1 | 180 |
| CTCCAGGGCACCATAGTCGTAG | |||
| Glutathione peroxidase (gpx) | CAGCAGACATTTCCTCACCATT | XM_038697220.1 | 250 |
| CAGTGGCAGAGTCAGCCTTTTA | |||
| Inhibitory protein of nuclear factor-kappa B (nfκb) | GCCAGAAGACAACCATACGCAT | XM_038729519.1 | 164 |
| GGACACCAGGAGACGCTCACAC | |||
| Nuclear factor-kappa B (iκb) | CACACTCGGTGATGATAACTGG | XM_038699792.1 | 182 |
| CTCCAGTAACGAGTAGTATGTA | |||
| β-actin | CTTTCCTCGGTATGGAGTCTTG | MH018565.1 | 386 |
| CAGTCGTTTGGGTTTGTAGCAG |
| A0 | A400 | A4000 | A8000 | |
|---|---|---|---|---|
| Growth performance | ||||
| IBW (%) | 3.33 ± 0.06 | 3.40 ± 0.05 | 3.43 ± 0.06 | 3.37 ± 0.07 |
| FBW (g) | 38.47 ± 0.54 a | 48.62 ± 1.51 b | 36.76 ± 0.72 a | 37.64 ± 0.77 a |
| WGR (%) a | 946.87 ± 60.51 a | 1287.39 ± 36.07 b | 903.13 ± 49.14 a | 895.64 ± 51.55 a |
| SGR (%/d) b | 3.53 ± 0.02 a | 3.85 ± 0.05 b | 3.43 ± 0.03 a | 3.49 ± 0.03 a |
| FE (%)c | 119.35 ± 7.5 a | 146.89 ± 3.16 b | 114.20 ± 5.45 a | 112.17 ± 4.57 a |
| Composition of whole fish | ||||
| Moisture | 72.16 ± 0.17 | 72.09 ± 0.26 | 71.36 ± 1.23 | 72.42 ± 0.23 |
| Ash | 3.54 ± 0.05 | 3.60 ± 0.02 | 3.70 ± 0.15 | 3.64 ± 0.04 |
| Crude lipid | 6.87 ± 0.29 | 6.97 ± 0.30 | 7.14 ± 0.30 | 6.54 ± 0.17 |
| Crude protein | 16.45 ± 0.08 | 16.38 ± 0.08 | 16.89 ± 0.78 | 16.17 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Wu, L.; Chen, Q.; Xu, W.; Li, D.; Han, D.; Zhu, X.; Liu, H.; Yang, Y.; Xie, S.; et al. Tolerance Assessment of Atractylodes macrocephala Polysaccharide in the Diet of Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 1581. https://doi.org/10.3390/antiox11081581
Dong B, Wu L, Chen Q, Xu W, Li D, Han D, Zhu X, Liu H, Yang Y, Xie S, et al. Tolerance Assessment of Atractylodes macrocephala Polysaccharide in the Diet of Largemouth Bass (Micropterus salmoides). Antioxidants. 2022; 11(8):1581. https://doi.org/10.3390/antiox11081581
Chicago/Turabian StyleDong, Bo, Liyun Wu, Qiaozhen Chen, Wenjie Xu, Dinggang Li, Dong Han, Xiaoming Zhu, Haokun Liu, Yunxia Yang, Shouqi Xie, and et al. 2022. "Tolerance Assessment of Atractylodes macrocephala Polysaccharide in the Diet of Largemouth Bass (Micropterus salmoides)" Antioxidants 11, no. 8: 1581. https://doi.org/10.3390/antiox11081581
APA StyleDong, B., Wu, L., Chen, Q., Xu, W., Li, D., Han, D., Zhu, X., Liu, H., Yang, Y., Xie, S., & Jin, J. (2022). Tolerance Assessment of Atractylodes macrocephala Polysaccharide in the Diet of Largemouth Bass (Micropterus salmoides). Antioxidants, 11(8), 1581. https://doi.org/10.3390/antiox11081581