Uptake and Immunomodulatory Properties of Betanin, Vulgaxanthin I and Indicaxanthin towards Caco-2 Intestinal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Materials and Samples
2.2. Cell Culture and Maintenance
2.3. Cytotoxicity Assay
2.4. Expression of Pro-Inflammatory and Antioxidant Markers
2.5. Quantification of Intracellular ROS Formation
2.6. Radical Assays and Electron Paramagnetic Resonance (EPR)
2.7. Trans-Epithelial Transport and Intracellular Accumulation of Purified Betalains
2.8. LC-MS Analysis
2.9. Statistics
3. Results and Discussion
3.1. Modulation of Pro-Inflammatory Cytokines by Betalains
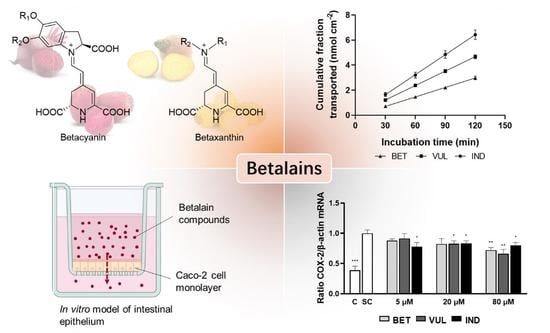
3.2. Betalain Effects on Expression of NF-κB Target Enzymes
3.3. Betalains Modulate the Expression of Nrf2 Target Enzymes
3.4. Effects on Intracellular Oxidative Stress and Radical Scavenging Activities
3.5. Intracellular Uptake of Purified Betalains
3.6. Trans-Epithelial Transport of Purified Betalains
3.7. Availability of Betalains, the Bottleneck to Immunomodulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.R.; Lennard, M.S.; Mason, S.L.; Tucker, G.T.; Woods, H.F. Beeturia and the biological fate of beetroot pigments. Pharmacogenetics 1993, 3, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Abdelfadeil, M.G.; Elbandy, M.A. Stability of Betalain Pigments from Red Beetroot (Beta vulgaris). Egypt. J. Food Sci. 2008, 36, 49–60. [Google Scholar]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from cactus pear fruit exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajka-Kuzniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.; Cano, M.P. In Vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087–128098. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Plant Betalains Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Toscano, G.; Cimino, F.; Saija, A. In Vitro Protective Effects of a Standardized Extract from Cynara cardunculus L. Leaves Against TNF-α-Induced Intestinal Inflammation. Front. Pharm. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.-J. Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, C.N.; Forbes, J.D. Gut Microbiome in Inflammatory Bowel Disease and Other Chronic Immune-Mediated Inflammatory Diseases. Inflamm. Intest. Dis. 2017, 2, 116–123. [Google Scholar] [CrossRef]
- Akbar, H.; Sadiq, Z.; Zia-Ul-Haq, M. Betalains: Biomolecular Aspects; Springer International Publishing AG: Cham, Switzerland, 2018; p. 193. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Sawicki, T.; Topolska, J.; Bączek, N.; Szawara-Nowak, D.; Juśkiewicz, J.; Wiczkowski, W. Characterization of the profile and concentration of betacyanin in the gastric content, blood and urine of rats after an intragastric administration of fermented red beet juice. Food Chem. 2020, 313, 126169–126178. [Google Scholar] [CrossRef]
- Takahashi, A.; Okumura, J.; Morita, Y.; Chiji, H. Intestinal Absorption and Antioxidant Activity of Betalain: A Nitrogen-containing Pigment from Table Beets and Cactus Pear Fruit Juice. J. Jpn. Soc. Food Sci. 2017, 64, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Wiczkowski, W.; Romaszko, E.; Szawara-Nowak, D.; Piskula, M.K. The impact of the matrix of red beet products and interindividual variability on betacyanins bioavailability in humans. Food Res. Int. 2018, 108, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Topolska, J.; Romaszko, E.; Wiczkowski, W. Profile and Content of Betalains in Plasma and Urine of Volunteers after Long-Term Exposure to Fermented Red Beet Juice. J. Agric. Food Chem. 2018, 66, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hithamani, G.; Kizhakayil, D.; Srinivasan, K. Uptake of phenolic compounds from plant foods in human intestinal Caco-2 cells. J. Biosci. 2017, 42, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Leach, D.N.; Wohlmuth, H.; De Voss, J.J.; Blanchfield, J.T. Caco-2 Cell Permeability of Flavonoids and Saponins from Gynostemma pentaphyllum: The Immortal Herb. ACS Omega 2020, 5, 21561–21569. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Aisling, S.A.; O’Brien, N.M. Investigation of beta-carotene and lutein transport in Caco-2 cells: Carotenoid-carotenoid interactions and transport inhibition by ezetimibe. Int. J. Vitam. Nutr. Res. 2009, 79, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.S.N.; Sergeeva, N.N.; Frutos, M.J.; Marshall, L.J.; Boesch, C. Novel approach for purification of major betalains using flash chromatography and comparison of radical scavenging and antioxidant activities. Food Chem. 2022, 385, 132632–132642. [Google Scholar] [CrossRef]
- Van De Walle, J.; Hendrickx, A.; Fau-Romier, B.; Romier, B.; Fau-Larondelle, Y.; Larondelle, Y.; Fau-Schneider, Y.-J.; Schneider, Y.J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. Vitr. 2010, 24, 1441–1449. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Effects of Uptake of Flavonoids on Oxidative Stress Induced by Hydrogen Peroxide in Human Intestinal Caco-2 Cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef]
- Ng, N.S.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio-Protoc. 2021, 11, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M. Regulation of tissue homeostasis by NF-κB signalling: Implications for inflammatory diseases. Nat. Rev. Immunol. 2009, 9, 778–788. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-kappaB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1beta-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Baggiolini, M.; Dewald, B.; Moser, B. lnterleukin-8 and Related Chemotactic Cytokines—CXC and CC Chemokines. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1993; Volume 55, pp. 97–179. [Google Scholar]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990, 4, 2860–2867. [Google Scholar] [CrossRef]
- Needleman, P.; Manning, P.T. Interactions between the inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) pathways: Implications for therapeutic intervention in osteoarthritis. Osteoarthr. Cartil. 1999, 7, 367–370. [Google Scholar] [CrossRef] [Green Version]
- MacDermott, R.P. Alterations of the mucosal immune system in inflammatory bowel disease. J. Gastroenterol. 1996, 31, 907–916. [Google Scholar] [CrossRef]
- Vignoli, A.L.; Srivastava, A.C.; Stammati, A.; Turco, L.; Tanori, M.; Zucco, F. Nitric oxide production in Caco-2 cells exposed to different inducers, inhibitors and natural toxins. Toxicol. Vitr. 2001, 15, 289–295. [Google Scholar] [CrossRef]
- Vidal, P.J.; López-Nicolás, J.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative Inhibition of Lipid Peroxidation, Cyclooxygenase Enzymes, and Human Tumor Cell Proliferation by Natural Food Colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef]
- Ahmadi, H.; Nayeri, Z.; Minuchehr, Z.; Sabouni, F.; Mohammadi, M. Betanin purification from red beetroots and evaluation of its anti-oxidant and anti-inflammatory activity on LPS-activated microglial cells. PLoS ONE 2020, 15, e0233088. [Google Scholar] [CrossRef]
- Chun, K.S.; Cha, H.H.; Shin, J.W.; Na, H.K.; Park, K.K.; Chung, W.Y.; Surh, Y.J. Nitric oxide induces expression of cyclooxygenase-2 in mouse skin through activation of NF-kappaB. Carcinogenesis 2004, 25, 445–454. [Google Scholar] [CrossRef]
- Vassalle, C.; Domenici, C.; Lubrano, V.; L’Abbate, A. Interaction between Nitric Oxide and Cyclooxygenase Pathways in Endothelial Cells. J. Vasc. Res. 2003, 40, 491–499. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Jovanovic, D.; Fernandes, J.C.; Manning, P.; Connor, J.R.; Currie, M.G.; Di Battista, J.A.; Martel-Pelletier, J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998, 41, 1275–1286. [Google Scholar] [CrossRef]
- Hu, Y.-P.; Peng, Y.-B.; Zhang, Y.-F.; Wang, Y.; Yu, W.-R.; Yao, M.; Fu, X.-J. Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury. Oxidative Med. Cell. Longev. 2017, 2017, 4123854–4123861. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.J.; Skouras, C.; Haugk, B.; Charnley, R.M. Keap1–Nrf2 signalling in pancreatic cancer. Int. J. Biochem. Cell Biol. 2015, 65, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-I.; Kang, J.; Stipanuk, M.H. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem. J. 2006, 393, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Pal, A.; Srivastava, S.K.; Orchard, J.L.; Singh, S.V. Differential expression of glutathione S-transferase isoenzymes in murine small intestine and colon. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 443–452. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant and Anti-Inflammatory Components of Foods; ILSI Europe: Brussels, Belgium, 2015; p. 34. [Google Scholar]
- Moreno-Ley, C.M.; Osorio-Revilla, G.; Hernández-Martínez, D.M.; Ramos-Monroy, O.A.; Gallardo-Velázquez, T. Anti-inflammatory activity of betalains: A comprehensive review. Hum. Nutr. Metab. 2021, 25, 200126–200132. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [Green Version]
- René, A.; Abasq, M.-L.; Hauchard, D.; Hapiot, P. How Do Phenolic Compounds React toward Superoxide Ion? A Simple Electrochemical Method for Evaluating Antioxidant Capacity. Anal. Chem. 2010, 82, 8703–8710. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 33–43. [Google Scholar] [CrossRef]
- Liveri, M.L.T.; Sciascia, L.; Lombardo, R.; Tesoriere, L.; Passante, E.; Livrea, M.A. Spectrophotometric Evidence for the Solubilization Site of Betalain Pigments in Membrane Biomimetic Systems. J. Agric. Food Chem. 2007, 55, 2836–2840. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Baumgartner, M.T. Betanidin pKa Prediction Using DFT Methods. ACS Omega 2020, 5, 13751–13759. [Google Scholar] [CrossRef]
- Medicine, N.L.O. Pubchem-Compound Summary Vulgaxanthin-I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vulgaxanthin-I (accessed on 6 August 2021).
- Turco Liveri, M.L.; Sciascia, L.; Allegra, M.; Tesoriere, L.; Livrea, M.A. Partition of Indicaxanthin in Membrane Biomimetic Systems. A Kinetic and Modeling Approach. J. Agric. Food Chem. 2009, 57, 10959–10963. [Google Scholar] [CrossRef]
- Tsakelidou, E.; Virgiliou, C.; Valianou, L.; Gika, H.G.; Raikos, N.; Theodoridis, G. Sample Preparation Strategies for the Effective Quantitation of Hydrophilic Metabolites in Serum by Multi-Targeted HILIC-MS/MS. Metabolites 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Travis, S.; Menzies, I. Intestinal permeability: Functional assessment and significance. Clin. Sci. 1992, 82, 471–488. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.; Rowland, M.; Warhurst, G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol.Cell Physiol. 2001, 281, 388–397. [Google Scholar] [CrossRef]
- Ménard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef]
- El-Kattan, A.; Varma, M. Oral Absorption, Intestinal Metabolism and Human Oral Bioavailability. Top. Drug Metab. 2012, 10, 31087. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fau-Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-Glucoside and Cyanidin Protect Against Intestinal Barrier Damage and 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis. J. Med. Food 2019, 23, 90–99. [Google Scholar] [CrossRef]
- Le Phuong Nguyen, T.; Fenyvesi, F.; Remenyik, J.; Homoki, J.R.; Gogolák, P.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Vasvári, G.; Vecsernyés, M.; et al. Protective Effect of Pure Sour Cherry Anthocyanin Extract on Cytokine-Induced Inflammatory Caco-2 Monolayers. Nutrients 2018, 10, 861. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation In Vitro and Ex Vivo. Food Chem. 2019, 276, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.Y.; Ong, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus). Food Sci. Biotechnol. 2019, 28, 1163–1169. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In Vitro Digestion of Betalainic Foods. Stability and Bioaccessibility of Betaxanthins and Betacyanins and Antioxidative Potential of Food Digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Cheok, A.K.F.; Rodriguez-Mateos, A.; George, T.W.; Caton, P.W. Effect of betalain (betanin) supplementation on endothelial dysfunction and vascular tone in human umbilical vascular endothelial cells. Proc. Nutr. Soc. 2018, 77, E174. [Google Scholar] [CrossRef]





| Betalains | MW (g mol−1) | HBD | HBA | tPSA (Å2) | Log P |
|---|---|---|---|---|---|
| BET | 551.48 | 9 | 14 | 246.55 | −2.50 |
| VUL | 340.29 | 5 | 9 | 173.59 | −1.46 |
| IND | 309.3 | 4 | 7 | 126.94 | −1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fernando, G.S.N.; Sergeeva, N.N.; Vagkidis, N.; Chechik, V.; Do, T.; Marshall, L.J.; Boesch, C. Uptake and Immunomodulatory Properties of Betanin, Vulgaxanthin I and Indicaxanthin towards Caco-2 Intestinal Cells. Antioxidants 2022, 11, 1627. https://doi.org/10.3390/antiox11081627
Wang Y, Fernando GSN, Sergeeva NN, Vagkidis N, Chechik V, Do T, Marshall LJ, Boesch C. Uptake and Immunomodulatory Properties of Betanin, Vulgaxanthin I and Indicaxanthin towards Caco-2 Intestinal Cells. Antioxidants. 2022; 11(8):1627. https://doi.org/10.3390/antiox11081627
Chicago/Turabian StyleWang, Yunqing, Ganwarige Sumali N. Fernando, Natalia N. Sergeeva, Nikolaos Vagkidis, Victor Chechik, Thuy Do, Lisa J. Marshall, and Christine Boesch. 2022. "Uptake and Immunomodulatory Properties of Betanin, Vulgaxanthin I and Indicaxanthin towards Caco-2 Intestinal Cells" Antioxidants 11, no. 8: 1627. https://doi.org/10.3390/antiox11081627
APA StyleWang, Y., Fernando, G. S. N., Sergeeva, N. N., Vagkidis, N., Chechik, V., Do, T., Marshall, L. J., & Boesch, C. (2022). Uptake and Immunomodulatory Properties of Betanin, Vulgaxanthin I and Indicaxanthin towards Caco-2 Intestinal Cells. Antioxidants, 11(8), 1627. https://doi.org/10.3390/antiox11081627