Antioxidant Effects of Dietary Supplements on Adult COVID-19 Patients: Why Do We Not Also Use Them in Children?
Abstract
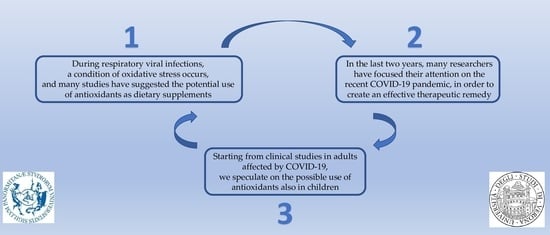
:1. Introduction
2. Methods
- Studies without full text available.
- Published studies in local languages, except for English.
- Non-relevant studies about other antioxidants, for the paucity of data in respiratory tract infections (i.e., copper, vitamin E, pentoxifylline).
- Studies about molecules that do not have a primary antioxidant role (i.e., vitamin D).
- Studies about the role of antioxidants in bacterial respiratory tract infections.
- Commentaries, letters and case-reports.
- In vitro studies about the interaction mechanism between coronavirus 2 and antioxidants.
- Clinical studies evaluating the potential role in preventing and/or treating SARS-CoV-2 infection of the main reviewed antioxidants in adults and children.
3. Role of Antioxidants in Prevention/Treatment of Coronavirus-19 Disease: Preclinical and Clinical Studies
3.1. Flavonoids
3.2. Quercetin
3.3. Hesperidin
3.4. Lactoferrin
3.5. Melatonin
3.6. Zinc and Selenium
3.7. Vitamins
3.8. Resveratrol
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Type of Antioxidant | Innate Immunity | Adaptive immunity |
|---|---|---|
| Flavonoids | Inhibition of ROS-generating enzymes and chelation of transition metal ions, which can catalyze ROS production [30] | Increase in lymphocyte proliferation and regulation of IFN-γ secretion [38]; reduction in IL-2 and IFN-γ release [45] |
| Quercetin | Inhibition of NF-κB activation; increase in neutrophil chemotaxis, NK-cells’ activity and macrophages’ phagocytosis [38] | Increase in lymphocyte proliferation and regulation of IFN-γ secretion [38] |
| Hesperidin | Reduction in TNF-α and IL-6 release [45] | Reduction in IL-2 and IFN-γ release [45] |
| Lactoferrin | Increase in neutrophils’ aggregation and NK-cells’ activity [48,49] Reduction in TNF-α and IL-6 serum levels [50] | Promotion of T and B-lymphocytes differentiation [49] |
| Melatonin | Inhibition of NF-κB activation [55] Suppression of NLRP3 inflammasome [55,56] | Increase in T-cells’ differentiation [56] |
| Zinc | Increase in neutrophils’ aggregation, macrophages’ phagocytosis and NK-cells’ activity [61] | Increase in T-cells’ differentiation, especially Th-1 cells [61,63] |
| Selenium | Increase in macrophages’ phagocytosis and NK-cells’ activity [61] Inhibition of NF-κB activation [69] Reduction in TNF-α and IL-6 serum levels [69] | Promotion of T-cells’ differentiation [61] Regulation of IFN-γ secretion [61] |
| Vitamin C | Increase in macrophages’ and neutrophils’activity [73,74,75,76] Promotion of NK-cells’ activity [73] Inhibition of NF-κB activation [73] | Promotion of T-cell differentiation, especially Th-1 and Th-17 cells [73,74,75,76] Promotion of antibody production [73] |
| Vitamin A | Promotion of dendritic cells’activity [84] Increase in macrophages’, NK-cells’ and neutrophils’activity [84] | Promotion of T-reg cell differentiation [82] Increase in IgAs production [82] |
| Resveratrol | Suppression of NLRP3 inflammasome [92] Inhibition of NF-κB activation [90,93] Reduction in IL-6 release [93] | Promotion of T-cell differentiation [92] |
References
- Chiappini, E.; Santamaria, F.; Marseglia, G.L.; Marchisio, P.; Galli, L.; Cutrera, R.; de Martino, M.; Antonini, S.; Becherucci, P.; Biasci, P.; et al. Prevention of recurrent respiratory infections. Inter-society Consensus. Ital. J. Pediatr. 2021, 47, 211. [Google Scholar] [CrossRef] [PubMed]
- Aykac, K.; Ozsurekci, Y.; Yayla, B.C.C.; Gurlevik, S.L.; Oygar, P.D.; Bolu, N.B.; Tasar, M.A.; Erdinc, F.S.; Ertem, G.T.; Neselioglu, S.; et al. Oxidant and antioxidant balance in patients with COVID-19. Pediatr. Pulmonol. 2021, 56, 2803–2810. [Google Scholar] [CrossRef]
- Graciano-Machuca, O.; Villegas-Rivera, G.; López-Pérez, I.; Macías-Barragán, J.; Sifuentes-Franco, S. Multisystem Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Role of Oxidative Stress. Front. Immunol. 2021, 19, 723654. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.S. Mild SARS-CoV-2 infections in children might be based on evolutionary biology and linked with host reactive oxidative stress and antioxidant capabilities. New Microbes New Infect. 2020, 36, 100723. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Myung Kong, J.; Hwang, Y.; Kang, J.S.; Lee, W.J. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef]
- Huang, S.H.; Cao, X.J.; Liu, W.; Shi, X.Y.; Wei, W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 2010, 48, 109–116. [Google Scholar] [CrossRef]
- Akhtar, S.; Das, J.K.; Ismail, T.; Wahid, M.; Saeed, W.; Bhutta, Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2021, 79, 289–300. [Google Scholar] [CrossRef]
- Di Stadio, A.; Della Volpe, A.; Korsch, F.M.; De Lucia, A.; Ralli, M.; Martines, F.; Ricci, G. Difensil Immuno Reduces Recurrence and Severity of Tonsillitis in Children: A Randomized Controlled Trial. Nutrients 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Junaid, K.; Ejaz, H.; Abdalla, A.E.; Abosalif, K.O.A.; Ullah, M.I.; Yasmeen Younas, S.; Hamam, S.S.M.; Rehman, A. Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients 2020, 12, 2992. [Google Scholar] [CrossRef] [PubMed]
- Victoni, T.; Barreto, E.; Lagente, V.; Carvalho, V.F. Oxidative Imbalance as a Crucial Factor in Inflammatory Lung Diseases: Could Antioxidant Treatment Constitute a New Therapeutic Strategy? Oxid Med. Cell. Longev. 2021, 2021, 6646923. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Harris, R.; Stout-Delgado, H.W. Targeted Antioxidants as Therapeutics for Treatment of Pneumonia in the Elderly. Transl. Res. 2020, 220, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Park, S.M.; Yang, S.R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of Thiols in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Liviero, F.; Campisi, M.; Mason, P.; Pavanello, S. Transient Receptor Potential Vanilloid Subtype 1: Potential Role in Infection, Susceptibility, Symptoms and Treatment of COVID-19. Front. Med. 2021, 8, 753819. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Wei, L.; Liu, Q.; Xie, M.; Weng, J.; Wang, X.; Chung, K.F.; Adcock, I.M.; Chen, Y.; et al. Role of TRPA1/TRPV1 in acute ozone exposure induced murine model of airway inflammation and bronchial hyperresponsiveness. J. Thorac. Dis. 2022, 14, 2698–2711. [Google Scholar] [CrossRef]
- Silva, R.A.P.; Chu, Y.; Miller, J.D.; Mitchell, I.J.; Penninger, J.M.; Faraci, F.M.; Heistad, D.D. Impact of ACE2 Deficiency and Oxidative Stress on Cerebrovascular Function with Aging. Stroke 2012, 43, 3358–3363. [Google Scholar] [CrossRef]
- Liao, M.T.; Wu, C.C.; Wu, S.F.V.; Lee, M.C.; Hu, W.C.; Tsai, K.W.; Yang, C.H.; Lu, C.L.; Chiu, S.K.; Lu, K.C. Resveratrol as an Adjunctive Therapy for Excessive Oxidative Stress in Aging COVID-19 Patients. Antioxidants 2021, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- De Ligt, M.; Hesselink, M.K.C.; Jorgensena, J.; Hoebersb, N.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Corrêa, C.B.; Rodrigues de Moura, T.; De Souza Araújo, A.A.; Martins-Filho, P.R.; Quintans-Júnior, L.J.; De Souza Siqueira Quintans, J. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Czarlewskic, W.; Zuberbiera, T.; Mullole, J.; Blainf, H.; Cristolg, J.P.; De La Torre, R.; Lozano, N.P.; Le Moing, V.; Bedbrook, A.; et al. Potential Interplay between Nrf2, TRPA1, and TRPV1 in Nutrients for the Control of COVID-19. Int. Arch. Allergy Immunol. 2021, 182, 324–338. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. DEFINE Study Investigators. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Gümüş, H.; Erat, T.; Öztürk, I.; Demir, A.; Koyuncu, I. Oxidative stress and decreased Nrf2 level in pediatric patients with COVID-19. J. Med. Virol. 2022, 94, 2259–2264. [Google Scholar] [CrossRef]
- Ducastel, M.; Chenevier-Gobeaux, C.; Ballaa, Y.; Meritet, J.F.; Brack, M.; Chapuis, N.; Pene, F.; Carlier, N.; Szwebel, T.A.; Roche, N.; et al. Oxidative Stress and Inflammatory Biomarkers for the Prediction of Severity and ICU Admission in Unselected Patients Hospitalized with COVID-19. Int. J. Mol. Sci. 2021, 22, 7462. [Google Scholar] [CrossRef]
- Lin, M.H.; Moses, D.C.; Hsieh, C.H.; Cheng, S.C.; Chen, Y.H.; Sun, C.Y.; Chou, C.Y. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 2018, 150, 155–163. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; La Maestra, S. Antioxidants and COVID-19. J. Prev. Med. Hyg. 2021, 62 (Suppl. 3), E34–E45. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Margolin, L.; Luchins, J.; Margolin, D.; Margolin, M.; Lefkowitz, S. 20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment. J. Evid. Based Integr. Med. 2021, 26, 2515690X211026193. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020, 286, 198070. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Paul, P.; Kumar, S.; Büsselberg, D.; Dwivedi, V.D.; Chaari, A. Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review. Int. J. Mol. Sci. 2021, 22, 11069. [Google Scholar] [CrossRef]
- Shawan, M.M.A.K.; Halder, S.K.; Hasan, M.A. Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An in silico molecular modeling approach in battling the COVID-19 outbreak. Bull. Natl. Res. Cent. 2021, 45, 27. [Google Scholar] [CrossRef]
- Anaya-Loyola, M.A.; García-Marín, G.; García-Gutiérrez, D.G.; Castaño-Tostado, E.; Reynoso-Camacho, R.; López-Ramos, J.E.; Enciso-Moreno, J.A.; Pérez-Ramírez, I.F. A mango (Mangifera indica L.) juice by-product reduces gastrointestinal and upper respiratory tract infection symptoms in children. Food. Res. Int. 2020, 136, 109492. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.X.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antiviral. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, I.E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health-The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 504–515. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wu, K.L.; Zhang, X.; Deng, S.Q.; Peng, B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020, 18, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Marín-Corral, J.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Pascual-Guardia, S.; Muñoz-Bermúdez, R.; Salazar-Degracia, A.; Pérez-Terán, P.; Restrepo, M.I.; Khymenets, O.; Haro, N.; et al. Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; El-Ashmawy, N.E.; Okasha, K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med. Hypotheses 2020, 144, 109957. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R. Age and Location in Severity of COVID-19 Pathology: Do Lactoferrin and Pneumococcal Vaccination Explain Low Infant Mortality and Regional Differences? Bioessays 2020, 42, e2000076. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as potential supplementary nutraceutical agent in COVID-19 patients: In vitro and in vivo preliminary evidences. bioRxiv 2020. [Google Scholar] [CrossRef]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study. Medicina 2021, 57, 842. [Google Scholar] [CrossRef]
- Molina-Carballo, A.; Jerez-Calero, A.E.; Muñoz-Hoyos, A. Possible Protective Role of Melatonin in Pediatric Infectious Diseases and Neurodevelopmental Pathologies. J. Child. Sci. 2020, 10, 104–109. [Google Scholar] [CrossRef]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef]
- Köken Yayici, Ö.; Gültutan, P.; Güngören, M.S.; Bayhan, G.I.; Yılmaz, D.; Gürkaş, E.; Özyürek, H.; Çitak Kurt, A.N. Impact of COVID-19 on serum melatonin levels and sleep parameters in children. Turk. J. Med. Sci. 2021, 51, 1640–1646. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Gouvarchin Ghaleh, H.E.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Arch. Med. Res. 2022, 53, 79–85. [Google Scholar] [CrossRef]
- Hasan, Z.T.; Al Atrakji, M.Q.Y.M.A.; Mehuaiden, A.K. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Int. J. Infect. Dis. 2022, 114, 79–84. [Google Scholar] [CrossRef]
- García García, I.; Rodriguez-Rubio, M.; Rodríguez Mariblanca, A.; Martínez de Soto, L.; Díaz García, L.; Monserrat Villatoro, J.; Queiruga Parada, J.; Seco Meseguer, E.; Rosales, M.J.; González, J.; et al. A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 466. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K.; Birdi, A.; Tomo, S.; Sreenivasulu, K.; Charan, J.; Yadav, D.; Purohit, P.; Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Ind. J. Clin. Biochem. 2021, 36, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Estevez, N.S.; Alvarez-Guevara, A.N.; Rodriguez-Martinez, C.E. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergol Immunopathol. 2016, 44, 368–375. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst.Rev. 2013, 31, CD000980. [Google Scholar] [CrossRef]
- Sánchez, J.; Villada, O.A.; Rojas, M.L.; Montoya, L.; Díaz, A.; Vargas, C.; Chica, J.; Herrera, A.M. Efecto del zinc aminoquelado y el sulfato de zinc en la incidencia de la infección respiratoria y la diarrea en niños preescolares de centros infantiles. Biomédica 2014, 34, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O. Therapeutic supplementation with zinc in the management of COVID-19-related diarrhea and ageusia/dysgeusia:mechanisms and clues for a personalized dosage regimen. Nutr. Rev. 2022, 80, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, S.; Soliman, S.; Esmail, E.S.; Khalaf, M.; Mostafa, E.F.; Medhat, M.A.; Ahmed, O.A.; Abd El Ghafar, M.S.; Alboraie, M.; Hassany, S.M. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine? A Randomized, Multicenter Trial. Biol. Trace Elem. Res. 2021, 199, 3642–3646. [Google Scholar] [CrossRef] [PubMed]
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Abdel-Aziz Soliman, M.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 2021, 93, 3176–3183. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Ghweil, A.A.; Hassan, M.H.; Rashad, A.; Khodeary, A.; Aref, Z.F.; Abdelrhman Sayed, M.A.; Elsamman, M.K.; Bazeed, S.E.S. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol. Trace Elem. Res. 2021, 199, 4101–4108. [Google Scholar] [CrossRef]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Medicie, M.D.S.G.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Shadvar, K.; Ostadi, Z.; Sanaie, S.; Saghaleini, S.H.; Nader, N.D. The Effect of Intravenous Selenium on Oxidative Stress in Critically III Patients with Acute Respiratory Distress Syndrome. Immunol. Invest. 2019, 48, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- Gómez, E.; Quidel, S.; Bravo-Soto, G.; Ortigoza, Á. Does vitamin C prevent the common cold? Medwave 2018, 18, e7235. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, W.; Wang, J.; Wang, H.; Zhao, Y.; Tseng, Y.; Bu, H. Extra Dose of Vitamin C Based on a Daily Supplementation Shortens the Common Cold: A Meta-Analysis of 9 Randomized Controlled Trials. Biomed Res. Int. 2018, 2018, 1837634. [Google Scholar] [CrossRef]
- Majidi, N.; Rabbani, F.; Gholami, S.; Gholamalizadeh, M.; BourBour, F.; Rastgoo, S.; Hajipour, A.; Shadnoosh, M.; Akbari, M.E.; Bahar, B. The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial. Front. Immunol. 2021, 12, 717816. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Saleh, K.B.; Badreldin, H.A.; Al Harthi, A.; Alenazi, M.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Alhammad, A.M. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: A propensity score matched study. Sci. Rep. 2021, 11, 17648. [Google Scholar] [CrossRef]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open. 2021, 4, e210369. [Google Scholar] [CrossRef] [PubMed]
- Chattha, K.S.; Kandasamy, S.; Vlasova, A.N.; Saif, L.J. Vitamin A deficiency impairs adaptive B and T cell responses to a prototype monovalent attenuated human rotavirus vaccine and virulent human rotavirus challenge in a gnotobiotic piglet model. PLoS ONE 2013, 8, e82966. [Google Scholar] [CrossRef]
- Jee, J.; Hoet, A.E.; Azevedo, M.P.; Vlasova, A.N.; Loerch, S.C.; Pickworth, C.L.; Hanson, J.; Saif, L.J. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am. J. Vet. Res. 2013, 74, 1353–1362. [Google Scholar] [CrossRef]
- McGill, J.L.; Kelly, S.M.; Guerra-Maupome, M.; Winkley, E.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019, 9, 15157. [Google Scholar] [CrossRef]
- Grotto, I.; Mimouni, M.; Gdalevich, M.; Mimouni, D. Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: A meta-analysis. J. Pediatr. 2003, 142, 297–304. [Google Scholar] [CrossRef]
- Amaral, C.T.; Pontes, N.N.; Maciel, B.L.L.; Bezerra, H.S.M.; Triesta, A.N.A.B.; Jeronimo, S.M.B.; McGowan, S.E.; Dantas, V.M. Vitamin A deficiency alters airway resistance in children with acute upper respiratory infection. Pediatr. Pulmonol. 2013, 48, 481–489. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, I.K.; Park, Y.J.; Kim, Y.S.; Kim, Y.J.; Chang, W.S.; Lee, Y.S.; Kweon, M.N.; Chung, Y.; Kang, C.Y. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 8742–8747. [Google Scholar] [CrossRef]
- Teran, R.; Mitre, E.; Vaca, M.; Erazo, S.; Oviedo, G.; Hübner, M.P.; Chico, M.E.; Mattapallil, J.J.; Bickle, Q.; Rodrigues, L.C.; et al. Immune system development during early childhood in tropical Latin America: Evidence for the age-dependent down regulation of the innate immune response. Clin. Immunol. 2011, 138, 299–310. [Google Scholar] [CrossRef]
- Midha, I.K.; Kumar, N.; Kumar, A.; Madan, T. Mega doses of retinol: A possible immunomodulation in Covid-19 illness in resource-limited settings. Rev. Med. Virol. 2021, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, A.M.; Capasso, M.; Della Volpe, A.; Malafronte, L.; Mansi, N.; Varricchio, A.; Ciprandi, G. Resveratrol plus carboxymethyl-β-glucan in children with recurrent respiratory infections: A preliminary and real-life experience. Ital. J. Pediatr. 2014, 40, 93. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother. Res. 2021, 35, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Sacco, O.; Capizzi, A.; Mastromarino, P. Can Resveratrol-Inhaled Formulations Be Considered Potential Adjunct Treatments for COVID-19? Front. Immunol. 2021, 12, 670955. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Di Mauro, A.; Labellarte, G.; Pignatelli, M.; Fanelli, M.; Schiavi, E.; Mastromarino, P.; Capozza, M.; Panza, R.; Laforgia, N. Resveratrol plus carboxymethyl-β-glucan in infants with common cold: A randomized double-blind trial. Heliyon 2020, 6, e03814. [Google Scholar] [CrossRef]

| Oxidative Stress Pattern | Mechanism of Action | SARS-CoV-2 Infection |
|---|---|---|
| ACE2 | It converts angiotensin II into angiotensin, with a consequent attenuation of inflammatory signaling cascades | The reduction in bioavailable ACE2 induces an overexpression of angiotensin II, which is able to activate OS patterns and inflammatory response |
| Nrf2 | It contributes to the expression of cell-protective genes in response to OS | The decrease in Nrf2 levels, through an increase in ROS production, might explain airway inflammation and tissue damage |
| TRPA1/TRPV1 | They are sensors of OS and are able to mediate airway inflammation and tissue injury | COVID-19 morbidity may be associated with TRPA1 and/or TRPV1 activation as well as desensitization |
| Thiols | They have antioxidant and anti-inflammatory properties | Reduced thiol plasma concentration has been correlated with severity of COVID19 |
| Type of Antioxidant | References | Country | Study Type | Study Population | Aim of the Study | Results |
|---|---|---|---|---|---|---|
| Quercetin | Shohan et al. [43] | Iran | Randomized controlled clinical trial | 60 adults (age > 18 years) affected by severe forms of COVID-19 who were not admitted to the ICU | Evaluating the therapeutic efficacy of quercetin administered in combination with antiviral drugs such as remdesivir and favipiravir in COVID-19 adult patients | Daily enteral administration of quercetin in addition to antiviral therapy results in a significant reduction in days of hospitalization following completion of therapy. Results close to statistical significance in the number of ICU admission days, number of deaths and number of discharges with benefit in the group of patients supplemented with quercetin. |
| Di Pierro et al. [44] | Italy | Randomized controlled open-label clinical trial | 42 patients affected by mild COVID-19 (age > 18 years) treated at home | Compare standard care with oral administration of quercetin in addition to conventional therapy in COVID-19 adult patients | Faster SARS-CoV-2 RT-PCR negativization and improvement of symptoms in patients supplemented with oral quercetin. | |
| Lactoferrin | Serrano et al. [49] | Spain | Prospective observational study | 75 adults (median age 42 years) affected by COVID-19 | Evaluating the therapeutic role of lactoferrin in SARS-CoV-2 infection and its preventative role in individuals in contact with symptomatic patients | The oral liposomal bovine lactoferrin administration at a therapeutic dose (64–96 mg every 6 h daily) in the first 5 days of infection caused improvement in respiratory symptoms (headache, muscular pain, taste, smell, weakness and dry cough) and a faster recovery compared to a control group. Individuals in contact with symptomatic patients, treated with half of the therapeutic dose, had a benefit in disease prevention. |
| Algahtani et al. [54] | Egypt | Randomized prospective interventional pilot study | 54 adults (median age 48 years) affected by COVID-19 | Evaluating the therapeutic role of lactoferrin in SARS-CoV-2 mild to moderate infection | Oral supplementation of lactoferrin is associated with an improvement of symptoms (fever, dry cough, diarrhea, headache, loss of sense of taste and/or smell and tiredness) after 7 days of lactoferrin treatment, although not statistically significant. At the same time, the improvement in laboratory markers (hemoglobin and albumin increase; liver enzymes, lactate dehydrogenase and C-reactive protein reduction) was not statistically significant in the treated groups compared to the control one. | |
| Melatonin | Farnoosh et al. [58] | Iran | Single-center double blind randomized clinical trial | 74 patients (>18 years old) affected by mild to moderate COVID-19 | Evaluating the efficacy and safety of oral melatonin in combination with standard treatment in adult hospitalized patients affected by COVID-19 | The administration of oral melatonin (3 mg, three times a day) combined with standard of care was correlated to an improvement in respiratory symptoms (cough, dyspnea) and fatigue. Moreover, in treated patients, there was an improvement also in laboratory (C-reactive protein serum levels) and radiologic (chest X-ray) exams. Finally, return to baseline health was significantly shorter in the patients receiving melatonin supplementation. |
| Hasan et al. [59] | Iraq | Randomized clinical trial | 158 patients (>18 and <80 years old) affected by mild to moderate COVID-19 | Evaluating the efficacy of oral melatonin in combination with standard treatment in adult hospitalized patients affected by COVID-19 | The administration of oral melatonin (10 mg/day), associated with standard of care, was more effective than standard of care alone in affected severe COVID-19 patients. In particular, it exerted a potential role in reduction of thrombosis events, sepsis onset and mortality rate in the treated group. | |
| Köken Yayici O et al. [57] | Turkey | Cross-sectional study | 84 patients (7–15 years and older) affected by COVID-19 | Evaluating serum melatonin concentration in children affected by SARS-CoV-2 infection | The study showed a lower concentration of melatonin in SARS-CoV-2 affected children in the 7–12 age group; no statistical difference in the other age groups. No changes in sleep patterns in affected children. | |
| Zinc | Abd-Elsalam et al. [66] | Egypt | Randomized controlled study | 191 patients (median age 43 years) affected by COVID-19 | Evaluating the efficacy of oral zinc supplementation in patients treated with chloroquine/hydroxychloroquine | Patients treated with oral zinc supplementation showed neither clinical nor laboratory improvements if compared to the control group (no statistically significant results). |
| Elalfy et al. [67] | Egypt | Non randomized controlled trial | 113 patients divided into two groups (<35 years and >35 years) affected by mild or moderate COVID-19 | Evaluating the oral synergistic effect of zinc when associated to a triple therapy (nitazoxanide, ribavirin and ivermectin) in COVID-19 affected patients | The combination of nitazoxanide, ribavirin and ivermectin plus zinc was effective in suppressing the shedding of SARS-CoV-2 in nasopharyngeal swabs compared to those receiving routine supportive symptomatic treatment alone. | |
| Abdelmaksoud et al. [68] | Egypt | Prospective clinical trial | 134 patients (median age 52 years) affected by mild to extremely severe COVID-19 | Evaluating how many patients had smell or taste disorders and, among these, how oral zinc supplementation could interfere with the median duration of complete recovery | The median duration of taste and/or smell recovery was significantly shorter among patients who received zinc therapy (220 mg zinc sulfate, equivalent to 50 mg elemental zinc, twice daily) than those who did not receive zinc, while the median duration of complete recovery from COVID-19 was not significantly different among the two groups. | |
| Selenium | Majeed et al. [71] | India | Cross-sectional study | 30 adults (18–45 years of age) affected by COVID-19 | Evaluating serum selenium concentration in adults affected by SARS-CoV-2 infection | The study showed a lower concentration of selenium in SARS-CoV-2 affected adults compared to the control group. |
| Ascorbic acid | Majidi et al. [79] | Iran | Randomized double-blind clinical trial | 120 adults (age range 35–75 years) affected by severe forms of COVID-19 with intensive support needs | Evaluating enteral administration of 500 mg ascorbic acid daily for 14 days in adults affected by COVID-19 | At day 14, a significant difference in survival was observed in favor of the ascorbic acid-supplemented patient group. |
| Al Sulaiman et al. [80] | Saudi Arabia | Two-center, non-interventional retrospective cohort study | 739 patients (≥18 years-old) affected by COVID-19, 158 of whom had been given ascorbic acid enterally | Evaluating enteral administration of ascorbic acid in COVID-19 adult patients | In the ascorbic acid-supplemented group there was no significant decrease in mortality, but there was a decrease in the incidence of thrombosis. | |
| Zhang J et al. [82] | China | Randomized controlled clinical trial | 56 patients aged 18–80 years affected by severe COVID-19 admitted to the ICU | Compare high-dose intravenous vitamin C (24 g/day) with placebo for 7 days | At the end of the observation period, there was no significant difference either on days free from invasive mechanical ventilation or 28-day mortality; an improvement in oxygenation was observed in vitamin C-treated patients and, similarly, a lower mean value of IL-6. | |
| Thomas et al. [83] | United States | Open-label randomized controlled clinical trial | 214 adult patients (18 years or older) affected by SARS-CoV-2 infection | Compare the administration of conventional therapy alone with the addition of either zinc gluconate or ascorbic acid or both for 10 days | Nonsignificant difference in the number of days needed to achieve a 50 percent reduction in symptoms. | |
| Resveratrol | De Ligt et al. [22] | Netherlands | Randomized double-blind placebo controlled crossover trial | 11 obese males (median age 53 years) not affected by COVID-19 | Evaluating the effects of 30-days resveratrol supplementation on renin–angiotensin–system components in the adipose tissue of otherwise healthy obese men | Resveratrol supplementation reduces ACE2 expression in human adipose tissue so, the prophylactic use of resveratrol in obese individuals could make them less susceptible for SARS-CoV-2 infection. Moreover, resveratrol reduces leptin serum levels in this population and its use could be beneficial as supplementary therapy in COVID-19 severe forms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarbartolo, V.; Montante, C.; Ferrante, G.; Giuffrè, M. Antioxidant Effects of Dietary Supplements on Adult COVID-19 Patients: Why Do We Not Also Use Them in Children? Antioxidants 2022, 11, 1638. https://doi.org/10.3390/antiox11091638
Notarbartolo V, Montante C, Ferrante G, Giuffrè M. Antioxidant Effects of Dietary Supplements on Adult COVID-19 Patients: Why Do We Not Also Use Them in Children? Antioxidants. 2022; 11(9):1638. https://doi.org/10.3390/antiox11091638
Chicago/Turabian StyleNotarbartolo, Veronica, Claudio Montante, Giuliana Ferrante, and Mario Giuffrè. 2022. "Antioxidant Effects of Dietary Supplements on Adult COVID-19 Patients: Why Do We Not Also Use Them in Children?" Antioxidants 11, no. 9: 1638. https://doi.org/10.3390/antiox11091638
APA StyleNotarbartolo, V., Montante, C., Ferrante, G., & Giuffrè, M. (2022). Antioxidant Effects of Dietary Supplements on Adult COVID-19 Patients: Why Do We Not Also Use Them in Children? Antioxidants, 11(9), 1638. https://doi.org/10.3390/antiox11091638