New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose
Abstract
:1. Introduction
2. Materials and Methods
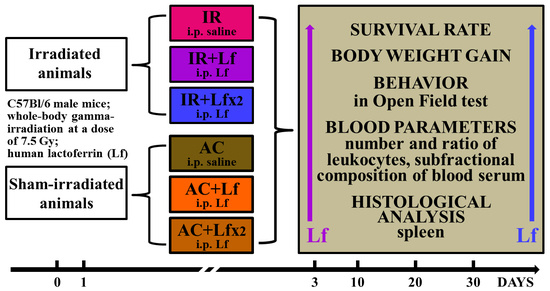
2.1. Experimental Groups and Treatments
2.2. Analysis of Mouse Behavior in the Open Field Test
2.3. Collection and Processing of Samples
2.3.1. Measurement of Hemoglobin Level
2.3.2. Leukocyte Counting
2.3.3. Differential Leukocyte Counting
2.3.4. Calculation of the Absolute Number of Leukocytes of Different Types
2.3.5. Dynamic Light Scattering Analysis of Blood Serum (DLS)
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Lf on Survival Rate and Lifespan of Mice Exposed to Irradiation
3.2. Effect of Lf on the Body Weight of Mice Exposed to Irradiation
3.3. Effects of Lf on Mouse Behavior in Open Field Test after Whole-Body Gamma Irradiation
3.4. Effects of Lf on Changes in Blood Parameters of Mice Exposed to Irradiation
3.4.1. Hemoglobin Level
3.4.2. Total Leukocyte Count
3.4.3. Differential Leukocyte Count
3.5. Effects of Lf on Changes in Subfraction Composition of the Blood Serum after Gamma Irradiation
3.6. Histological Analysis of Mouse Spleen and Liver after Total Gamma Irradiation
3.6.1. Spleen
3.6.2. Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seed, T.M. Radiation protectants: Current status and future prospects. Health Phys. 2005, 89, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. Radioprotection by Antioxidantsa. Ann. N. Y. Acad. Sci. 2006, 899, 44–60. [Google Scholar] [CrossRef]
- Nishimura, Y.; Homma-Takeda, S.; Kim, H.-S.; Kakuta, I. Radioprotection of Mice by Lactoferrin against Irradiation with Sublethal X-rays. J. Radiat. Res. 2014, 55, 277–282. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Imase, M.; Oda, H.; Wakabayashi, H.; Ishii, K. Lactoferrin Directly Scavenges Hydroxyl Radicals and Undergoes Oxidative Self-Degradation: A Possible Role in Protection against Oxidative DNA Damage. Int. J. Mol. Sci. 2014, 15, 1003–1013. [Google Scholar] [CrossRef]
- Ward, P.P.; Conneely, O.M. Lactoferrin: Role in Iron Homeostasis and Host Defense against Microbial Infection. BioMetals 2004, 17, 203–208. [Google Scholar] [CrossRef]
- Johnson, E.E.; Wessling-Resnick, M. Iron Metabolism and the Innate Immune Response to Infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef]
- Orsi, N. The Antimicrobial Activity of Lactoferrin: Current Status and Perspectives. BioMetals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin: Mammalian Lactoferrin Receptors: Structure and Function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of Lactoferrin with Cells Involved in Immune FunctionThis Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 7th International Conference on Lactoferrin: Structure, Function, and Applications, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [CrossRef]
- Leveugle, B.; Mazurier, J.; Legrand, D.; Mazurier, C.; Montreuil, J.; Spik, G. Lactotransferrin Binding to Its Platelet Receptor Inhibits Platelet Aggregation. Eur. J. Biochem. 1993, 213, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Bennatt, D.J.; McAbee, D.D. Identification and Isolation of a 45-KDa Calcium-Dependent Lactoferrin Receptor from Rat Hepatocytes. Biochemistry 1997, 36, 8359–8366. [Google Scholar] [CrossRef]
- Takayama, Y.; Takahashi, H.; Mizumachi, K.; Takezawa, T. Low Density Lipoprotein Receptor-Related Protein (LRP) Is Required for Lactoferrin-Enhanced Collagen Gel Contractile Activity of Human Fibroblasts. J. Biol. Chem. 2003, 278, 22112–22118. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Banovic, T.; Zhu, Q.; Watson, M.; Callon, K.; Palmano, K.; Ross, J.; Naot, D.; Reid, I.R.; Cornish, J. The Low-Density Lipoprotein Receptor-Related Protein 1 Is a Mitogenic Receptor for Lactoferrin in Osteoblastic Cells. Mol. Endocrinol. 2004, 18, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-Mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules. 2020, 10, 456. [Google Scholar] [CrossRef]
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Hayes, T.G.; Falchook, G.F.; Varadhachary, G.R.; Smith, D.P.; Davis, L.D.; Dhingra, H.M.; Hayes, B.P.; Varadhachary, A. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Investig. New Drugs. 2006, 24, 233–240. [Google Scholar] [CrossRef]
- Troost, F.J.; Saris, W.H.; Brummer, R.J. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur. J. Clin. Nutr. 2003, 57, 1579–1585. [Google Scholar] [CrossRef]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W., Jr.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer. 2004, 111, 398–403. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsuura, M.; Kobayashi, K.; Sasaki, H.; Yajima, T.; Kuwata, T. Lactoferrin protects against development of hepatitis caused by sensitization of Kupffer cells by lipopolysaccharide. Clin. Diagn. Lab. Immunol. 2001, 8, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. GRN 000456 Cow’s Milk-Derived Lactoferrin; U.S. Food and Drug Administration’s Office of Food Additive Safety: Silver Spring, MD, USA, 2016. Available online: https://www.fda.gov/media/153787/download (accessed on 9 September 2022).
- Feng, L.; Li, J.; Qin, L.; Guo, D.; Ding, H.; Deng, D. Radioprotective Effect of Lactoferrin in Mice Exposed to Sublethal X-ray Irradiation. Exp. Ther. Med. 2018, 16, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-L.; Xu, J.-Y.; Zhang, R.; Zhang, Z.; Zhao, L.; Qin, L.-Q. Effects of Lactoferrin on X-Ray-Induced Intestinal Injury in Balb/C Mice. Appl. Radiat. Isot. 2019, 146, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Caring for Animals Aiming for Better Science. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/guidance/directive/en.pdf (accessed on 28 July 2022).
- Faraji, N.; Zhang, Y.; Ray, A.K. Determination of Adsorption Isotherm Parameters for Minor Whey Proteins by Gradient Elution Preparative Liquid Chromatography. J. Chromatogr. A 2015, 1412, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Hassan, M.I.; Kashav, T.; Singh, T.P.; Yadav, S. Heparin-Binding Proteins of Human Seminal Plasma: Purification and Characterization. Mol. Reprod. Dev. 2008, 75, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, F.; Wu, W.; Dong, X.; Ren, J.; Shen, Q. Determination of Bovine Lactoferrin in Food by HPLC with a Heparin Affinity Column for Sample Preparation. J. AOAC Int. 2017, 100, 133–138. [Google Scholar] [CrossRef]
- Alchinova, I.B.; Polyakova, M.V.; Yakovenko, E.N.; Medvedeva, Y.S.; Saburina, I.N.; Karganov, M.Y. Effect of Extracellular Vesicles Formed by Multipotent Mesenchymal Stromal Cells on Irradiated Animals. Bul.l Exp. Biol. Med. 2019, 166, 574–579. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Hao, Y.; Gu, Y.; Yang, Z.; Li, H.; Li, R. 6,7,3′,4′-Tetrahydroxyisoflavone Improves the Survival of Whole-Body-Irradiated Mice via Restoration of Hematopoietic Function. Int. J. Radiat. Biol. 2017, 93, 793–802. [Google Scholar] [CrossRef]
- Kopaeva, Y.; Cherepov, A.B.; Zarayskaya, I.Y.; Nesterenko, M.V. Transport of Human Lactoferrin into Mouse Brain: Administration Routes and Distribution. Bull. Exp. Biol. Med. 2019, 167, 561–567. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Kopaeva, M.Y.; Alchinova, I.B.; Nesterenko, M.V.; Cherepov, A.B.; Demorzhi, M.S.; Zarayskaya, I.Y.; Karganov, M.Y. Radioprotective effect of human lactoferrin against gamma-irradiation with sublethal dose. In Proceedings of the RAD Conference Proceedings. 20–24 Aug. 2020, Herceg Novi, Montenegro; RAD Centre: Nis, Serbia, 2020; Volume 4, pp. 45–49. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. ISBN 978-1-60761-302-2. [Google Scholar]
- Karganov, M.; Alchinova, I.; Arkhipova, E.; Skalny, A.V. Laser Correlation Spectroscopy: Nutritional, Ecological and Toxic Aspects. In Biophysics; Misra, A.N., Ed.; InTech: London, UK, 2012; pp. 1–16. ISBN 978-953-51-0376-9. [Google Scholar]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied, 4th ed.; Churchill Livingstone: Edinburgh, UK; London, UK; New York, NY, USA, 1980; Volume 1. [Google Scholar]
- Mandillo, S.; Tucci, V.; Hölter, S.M.; Meziane, H.; Al, M.; Kallnik, M.; Lad, H.V.; Nolan, P.M.; Ouagazzal, A.-M.; Coghill, E.L.; et al. Reliability, Robustness, and Reproducibility in Mouse Behavioral Phenotyping: A Cross-Laboratory Study. Physiol. Genomics. 2008, 34, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the Elevated Plus-Maze and Open-Field Tests for the Assessment of Anxiety-Related Behaviour in Inbred Mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Bella, L.M.; Fieri, I.; Tessaro, F.H.G.; Nolasco, E.L.; Nunes, F.P.B.; Ferreira, S.S.; Azevedo, C.B.; Martins, J.O. Vitamin D Modulates Hematological Parameters and Cell Migration into Peritoneal and Pulmonary Cavities in Alloxan-Diabetic Mice. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, M.F.; Akleev, A.V.; Pashkov, I.A.; Klopov, N.V.; Noskin, V.A.; Noskin, L.A. Lazernaia korreliatsionnaia spektroskopiia plazmy krovi dlia diagnostiki postradiatsionnykh posledstviĭ [Laser correlation spectroscopy of blood plasma for diagnosis of postradiation sequels]. Radiats Biol. Radioecol. 1999, 39, 64–78. (In Russian) [Google Scholar] [PubMed]
- Alchinova, I.B.; Arkhipova, E.N.; Medvedeva, Y.S.; Cherepov, A.B.; Karganov, M.Y. Dynamics of Changes in Physiological Parameters of Mice with Different Radiosensitivity after Acute γ-Irradiation. Bull. Exp. Biol. Med. 2014, 157, 190–193. [Google Scholar] [CrossRef]
- Alchinova, I. The Complex of Tests for the Quantitative Evaluation of the Effects of Radiation on Laboratory Animals. Am. J. Life Sci. 2015, 3, 5. [Google Scholar] [CrossRef]
- Wang, S.; Lee, K.; Hyun, J.; Lee, Y.; Kim, Y.; Jung, Y. Hedgehog Signaling Influences Gender-Specific Response of Liver to Radiation in Mice. Hepatol. Int. 2013, 7, 1065–1074. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Li, D.; Song, N.; Yang, F.; Xu, W. MST1/2 Inhibitor XMU-MP-1 Alleviates the Injury Induced by Ionizing Radiation in Haematopoietic and Intestinal System. J. Cell. Mol. Med. 2022, 26, 1621–1628. [Google Scholar] [CrossRef]
- Der Meeren, A.V.; Lebaron-Jacobs, L. Behavioural Consequences of an 8 Gy Total Body Irradiation in Mice: Regulation by Interleukin-4. Can. J. Physiol. Pharmacol. 2001, 79, 140–143. [Google Scholar] [CrossRef]
- Kumar, V.P.; Holmes-Hampton, G.P.; Biswas, S.; Stone, S.; Sharma, N.K.; Hritzo, B.; Guilfoyle, M.; Eichenbaum, G.; Guha, C.; Ghosh, S.P. Mitigation of Total Body Irradiation-Induced Mortality and Hematopoietic Injury of Mice by a Thrombopoietin Mimetic (JNJ-26366821). Sci. Rep. 2022, 12, 3485. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Ulanova, A.M.; Stavrakova, N.M.; Deshevoĭ, I.B.; Nasonova, T.A.; Koterov, A.N.; Gutsenko, K.K.; Mal’tsev, V.N. Antiradiation effects of Lactoferrin. Radiatsionnaia Biol. Radioecol. Radiat. Biol. Radioecol. 2009, 49, 456–461. (In Russian) [Google Scholar]
- Maier, D.M.; Landauer, M.R.; Davis, H.D.; Walden, T.L. Effect of electron radiation on aggressive behavior, activity, and hemopoiesis in mice. J Radiat Res. 1989, 30, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.A.; Dalla Corte, C.L.; Andrade, E.R.; Marina, R.; González, P.; Barrio, J.P. Purple grape juice as a protector against acute x-irradiation induced alterations on mobility, anxiety, and feeding behaviour in mice. Nutr. Hosp. 2014, 29, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Wang, L.-M.; Yi, L.-R.; Jia, C.; Bai, F.; Peng, R.-J.; Yu, Z.-Y.; Xiong, G.-L.; Xing, S.; Shan, Y.-J.; et al. Succinate Ester Derivative of δ-Tocopherol Enhances the Protective Effects against 60Co γ-Ray-Induced Hematopoietic Injury through Granulocyte Colony-Stimulating Factor Induction in Mice. Sci. Rep. 2017, 7, 40380. [Google Scholar] [CrossRef] [PubMed]
- Erexson, G.L.; Kligerman, A.D.; Bryant, M.F.; Sontag, M.R.; Halperin, E.C. Induction of Micronuclei by X-Radiation in Human, Mouse and Rat Peripheral Blood Lymphocytes. Mutat. Res. Mutagen. Relat. Subj. 1991, 253, 193–198. [Google Scholar] [CrossRef]
- Xue, J.; Du, R.; Ling, S.; Song, J.; Yuan, X.; Liu, C.; Sun, W.; Li, Y.; Zhong, G.; Wang, Y.; et al. Osteoblast Derived Exosomes Alleviate Radiation- Induced Hematopoietic Injury. Front. Bioeng. Biotechnol. 2022, 10, 850303. [Google Scholar] [CrossRef]
- Christensen, D.M.; Iddins, C.J.; Sugarman, S.L. Ionizing Radiation Injuries and Illnesses. Emerg. Med. Clin. North Am. 2014, 32, 245–265. [Google Scholar] [CrossRef]
- Wolber, F.M.; Leonard, E.; Michael, S.; Orschell-Traycoff, C.M.; Yoder, M.C.; Srour, E.F. Roles of Spleen and Liver in Development of the Murine Hematopoietic System. Exp. Hematol. 2002, 30, 1010–1019. [Google Scholar] [CrossRef]
- Golden-Mason, L.; O’Farrelly, C. Having It All? Stem Cells, Haematopoiesis and Lymphopoiesis in Adult Human Liver. Immunol. Cell Biol. 2002, 80, 45–51. [Google Scholar] [CrossRef]
- Kim, C. Homeostatic and Pathogenic Extramedullary Hematopoiesis. J. Blood Med. 2010, 1, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Peslak, S.A.; Wenger, J.; Bemis, J.C.; Kingsley, P.D.; Koniski, A.D.; McGrath, K.E.; Palis, J. EPO-Mediated Expansion of Late-Stage Erythroid Progenitors in the Bone Marrow Initiates Recovery from Sublethal Radiation Stress. Blood 2012, 120, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Masutani, M.; Nozaki, T.; Nakamoto, K.; Nakagama, H.; Suzuki, H.; Kusuoka, O.; Tsutsumi, M.; Sugimura, T. The Response of Parp Knockout Mice against DNA Damaging Agents. Mutat. Res. Mutat. Res. 2000, 462, 159–166. [Google Scholar] [CrossRef]
- Volinsky, E.; Lazmi-Hailu, A.; Cohen, N.; Adani, B.; Faroja, M.; Grunewald, M.; Gorodetsky, R. Alleviation of Acute Radiation-Induced Bone Marrow Failure in Mice with Human Fetal Placental Stromal Cell Therapy. Stem Cell Res. Ther. 2020, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver Immunology and Its Role in Inflammation and Homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver Regeneration: Biological and Pathological Mechanisms and Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Selzner, N.; Selzner, M.; Odermatt, B.; Tian, Y.; van Rooijen, N.; Clavien, P. ICAM-1 Triggers Liver Regeneration through Leukocyte Recruitment and Kupffer Cell–Dependent Release of TNF-α/IL-6 in Mice. Gastroenterology 2003, 124, 692–700. [Google Scholar] [CrossRef]
- Birgens, H.S.; Hansen, N.E.; Karle, H.; Kristensen, L.Ø. Receptor Binding of Lactoferrin by Human Monocytes. Br. J. Haematol. 1983, 54, 383–391. [Google Scholar] [CrossRef]
- Crouch, S.; Slater, K.; Fletcher, J. Regulation of Cytokine Release from Mononuclear Cells by the Iron- Binding Protein Lactoferrin. Blood 1992, 80, 235–240. [Google Scholar] [CrossRef]
- Yin, H.; Cheng, L.; Holt, M.; Hail, N.; MacLaren, R.; Ju, C. Lactoferrin Protects against Acetaminophen-Induced Liver Injury in Mice. Hepatology 2010, 51, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A Review. Vet. Med. 2008, 53, 457–468. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol. Neurobiol. 2015, 52, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Determinants of Milk Lactoferrin as a Bioactive Compound in Early Neurodevelopment and Cognition. J. Pediatr. 2016, 173, S29–S36. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Azieva, A.M.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Human lactoferrin enhances the expression of transcription factor c-Fos in neuronal cultures under stimulated conditions. Nauchno-Prakt. Zhurnal Patogenez 2021, 19, 74–78. [Google Scholar] [CrossRef]
- Zimecki, M.; Mazurier, J. Human Lactoferrin Induces Phenotypic and Functional Changes in Murine Splenic B Cells. Immunology 1995, 86, 122–127. [Google Scholar]
- Legrand, D.; Vigie, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.-C.; Carpentier, M.; Briand, J.-P.; Mazurier, J.; Hovanessian, A.G. Surface Nucleolin Participates in Both the Binding and Endocytosis of Lactoferrin in Target Cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef]
- Jia, W.; Yao, Z.; Zhao, J.; Guan, Q.; Gao, L. New Perspectives of Physiological and Pathological Functions of Nucleolin (NCL). Life Sci. 2017, 186, 1–10. [Google Scholar] [CrossRef]















| Group | Day 3 | ||||
|---|---|---|---|---|---|
| Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils | |
| IR | 0.24 ± 0.02 * | 0.09 ± 0.02 * | 0.06 ± 0.02 * | 0.03 ± 0.01 * | 0.07 ± 0.02 * |
| IR+Lf | 0.41 ± 0.03 * | 0.19 ± 0.03 * | 0.07 ± 0.02 * | 0.03 ± 0.01 * | 0.07 ± 0.02 * |
| IR+Lf×2 | 0.39 ± 0.03 * | 0.23 ± 0.01 * | 0.06 ± 0.03 * | 0.03 ± 0.01 * | 0.05 ± 0.01 * |
| AC | 1.85 ± 0.20 | 7.02 ± 0.39 | 0.80 ± 0.11 | 0.17 ± 0.03 | 0.97 ± 0.15 |
| AC+Lf | 2.38 ± 0.21 | 7.07 ± 0.41 | 1.33 ± 0.20 | 0.21 ± 0.05 | 1.21 ± 0.05 |
| AC+Lf×2 | 2.01 ± 0.16 | 6.81 ± 0.11 | 1.00 ± 0.09 | 0.25 ± 0.04 | 1.12 ± 0.09 |
| Group | Day 30 | ||||
|---|---|---|---|---|---|
| Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils | |
| IR | 1.22 ± 0.12 * | 2.54 ± 0.29 * | 0.91 ± 0.32 | 0.13 ± 0.02 | 0.69 ± 0.12 * |
| IR+Lf | 0.90 ± 0.10 | 4.05 ± 0.24 * | 0.84 ± 0.16 | 0.18 ± 0.04 | 0.56 ± 0.05 * |
| IR+Lf×2 | 1.34 ± 0.11 | 5.79 ± 0.36 | 0.87 ± 0.16 | 0.14 ± 0.03 | 1.00 ± 0.16 |
| AC | 0.97 ± 0.08 | 6.90 ± 0.23 | 0.43 ± 0.09 | 0.16 ± 0.01 | 1.07 ± 0.13 |
| AC+Lf | 1.03 ± 0.14 | 5.91 ± 0.27 | 0.70 ± 0.13 | 0.14 ± 0.02 | 1.00 ± 0.08 |
| AC+Lf×2 | 1.28 ± 0.17 | 6.06 ± 0.31 | 0.54 ± 0.16 | 0.22 ± 0.04 | 1.11 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopaeva, M.Y.; Alchinova, I.B.; Cherepov, A.B.; Demorzhi, M.S.; Nesterenko, M.V.; Zarayskaya, I.Y.; Karganov, M.Y. New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose. Antioxidants 2022, 11, 1833. https://doi.org/10.3390/antiox11091833
Kopaeva MY, Alchinova IB, Cherepov AB, Demorzhi MS, Nesterenko MV, Zarayskaya IY, Karganov MY. New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose. Antioxidants. 2022; 11(9):1833. https://doi.org/10.3390/antiox11091833
Chicago/Turabian StyleKopaeva, Marina Yu., Irina B. Alchinova, Anton B. Cherepov, Marina S. Demorzhi, Mikhail V. Nesterenko, Irina Yu. Zarayskaya, and Mikhail Yu. Karganov. 2022. "New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose" Antioxidants 11, no. 9: 1833. https://doi.org/10.3390/antiox11091833
APA StyleKopaeva, M. Y., Alchinova, I. B., Cherepov, A. B., Demorzhi, M. S., Nesterenko, M. V., Zarayskaya, I. Y., & Karganov, M. Y. (2022). New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose. Antioxidants, 11(9), 1833. https://doi.org/10.3390/antiox11091833