The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish
Abstract
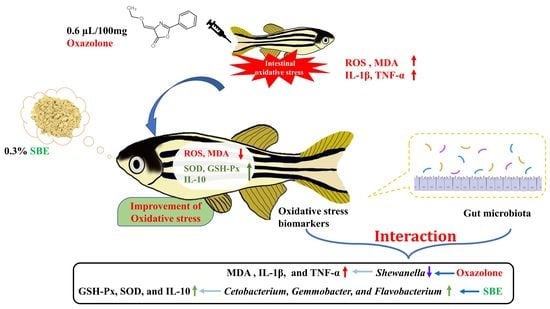
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract Preparation
2.3. Animal Experiments
2.4. Measurement of Oxidative Stress Biochemical Markers
2.5. Histological Analysis
2.6. Detection of Primary Active Substances
2.7. RNA Isolation and Real-Time Polymerase Chain Reaction
2.8. 16s rDNA Gene Sequencing
2.9. Data and Statistical Analysis
3. Results
3.1. Oxazolone-Induced Intestinal Oxidative Stress in Zebrafish
3.2. Antioxidant Effects of SBE and Main Active Substances
3.2.1. Effects of SBE on Intestinal Tissue Morphology
3.2.2. Effects of SBE on Biomarkers of Oxidative Stress
3.2.3. Main Active Substances of SBE
3.3. Effects of SBE on Intestinal Inflammatory Factors under Oxidative Stress
3.4. Effects of SBE on Intestinal Microbiota in Zebrafish
3.5. Effect of the Association between the Biomarkers of Oxidative Stress and Intestinal Microbiota in Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Barboza, G.D.; Guizzardi, S.; Moine, L.; de Talamoni, N.T. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemele, E.J.; Fulton, D.J.R.; Fukai, T. Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Zahari, A.; Ablat, A.; Sivasothy, Y.; Mohamad, J.; Choudhary, M.I.; Awang, K. In vitro antiplasmodial and antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri Kosterm. Asian Pac. J. Trop. Med. 2016, 9, 328–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ktari, N.; Bkhairia, I.; Nasri, R.; Kolsi, R.B.A.; Salem, R.B.S.B.; Amara, I.B.; Zeghal, N.; Salah, B.B.; Salah, R.B.; Nasri, M. Zebra blenny protein hydrolysates as a source of bioactive peptides with prevention effect against oxidative dysfunctions and DNA damage in heart tissues of rats fed a cholesterol-rich diet. Food Res Int. 2017, 100, 423–432. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Moura, F.A.; De Andrade, K.Q.; Dos Santos, J.C.F.; Araujo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.X.; Song, Y.; Zeng, C.X.; Zhang, H.W.; Lv, C.H.; Shi, M.; Qin, S. Molecular Mechanism Underlying the Regulatory Effect of Vine Tea on Metabolic Syndrome by Targeting Redox Balance and Gut Microbiota. Front. Nutr. 2022, 9, 802015. [Google Scholar] [CrossRef]
- Wang, Y.H. Current progress of research on intestinal bacterial translocation. Microb. Pathog. 2021, 152, 104652. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Veld, J.H.J.H.I. Overview of gut flora and probiotic. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Weng, G.Y.; Duan, Y.H.; Zhong, Y.Z.; Song, B.; Zheng, J.; Zhang, S.Y.; Yin, Y.L.; Deng, J.P. Plant Extracts in Obesity: A Role of Gut Microbiota. Front. Nutr. 2021, 8, 727951. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, H.; Lu, X.X.; Ling, L.J.; Weng, H.B.; Sun, W.; Chen, D.F.; Zhang, Y.Y. Houttuynia cordata polysaccharide alleviated intestinal injury and modulated intestinal microbiota in H1N1 virus infected mice. Chin. J. Nat. Med. 2019, 17, 187–197. [Google Scholar] [CrossRef]
- Lyu, Y.F.; Lin, L.; Xie, Y.N.; Li, D.; Xiao, M.; Zhang, Y.F.; Cheung, S.C.K.; Shaw, P.C.; Yang, X.; Chan, P.K.S.; et al. Blood-Glucose-Lowering Effect of Coptidis Rhizoma Extracts From Different Origins via Gut Microbiota Modulation in db/db Mice. Front. Pharmacol. 2021, 12, 684358. [Google Scholar] [CrossRef]
- Pan, F.F.; Zhang, H.Y.; Li, X.M.; Yang, P.L.; Zhang, T.C.; Luo, X.G.; Ma, W.J. Effect of quality control on the total antioxidant capacity of the extract from Sonchus brachyotus DC. Int. J. Food Prop. 2018, 21, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.X.; Liu, J.J.; Su, Y.; Li, M.L.; Xie, X.Y.; Su, J.J. Complete Chloroplast Genome Sequence of Sonchus brachyotus Helps to Elucidate Evolutionary Relationships with Related Species of Asteraceae. BioMed Res. Int. 2021, 2021, 9410496. [Google Scholar] [CrossRef] [PubMed]
- Qie, P.J.; Duan, X.C.; Wang, M.; Li, X.Z. Antioxidant activity of each polar composition from methanol extracts of Sonchus brachyotus DC. Sci. Technol. Food Ind. 2016, 36, 146–156. [Google Scholar]
- Xia, D.Z.; Yu, X.F.; Zhu, Z.Y.; Zou, Z.D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.F.; Li, X.M.; Zhang, H.Y.; Ma, W.J.; Yang, P.L. Optimization of Extracting Process of Total Alkaloids from Sonchus brachyotus DC. by Response Surface Method. Sci. Technol. Food Ind. 2018, 19, 194–199. [Google Scholar]
- Brugman, S.; Liu, K.Y.; Lindenbergh-Kortleve, D.; Samsom, J.N.; Furuta, G.T.; Renshaw, S.A.; Willemsen, R.; Nieuwenhuis, E.E. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 2009, 137, 1757–1767.e1. [Google Scholar] [CrossRef]
- Aliota, M.T.; Dudley, D.M.; Newman, C.M.; Weger-Lucarelli, J.; Stewart, L.M.; Koenig, M.R.; Breitbach, M.E.; Weiler, A.M.; Semler, M.R.; Barry, G.L.; et al. Molecularly barcoded Zika virus libraries to probe in vivo evolutionary dynamics. PLoS Pathog. 2018, 14, e1006964. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.J.; Ma, S.J.; Jiao, C.W.; Yang, Y.S.; Chen, J.M.; Li, L.Q.; Xie, Y.Z. Optimization on Determination Method for Polysaccharides Content of Ganoderma lucidum Spore Powder. Edible Fungi China 2018, 37, 67–72. [Google Scholar] [CrossRef]
- Ren, M.y.; Wang, S.S.; Gao, H.; Yu, H.K.; Chen, L.; Wang, B.Y.; Yang, Y.C. Extraction and Antioxidants of polyphenol from Black Soyben. Modern Food. 2018, 19, 91–94. [Google Scholar] [CrossRef]
- Wang, J.; Deng, C.C.; Xu, Z.Q.; Bi, Y.; Wang, Z.Z. Establishment of content determination and optimization extraction condition of total alkaloids in Maca. Sci. Technol. Food Ind. 2019, 35, 302–310. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Liu, J.X.; Zhang, Y.F.; Qiao, H.Z.; Chen, F.; Zhang, S.H.; Guan, W.T. Anethole Attenuates Enterotoxigenic Escherichia coli-Induced Intestinal Barrier Disruption and Intestinal Inflammation via Modification of TLR Signaling and Intestinal Microbiota. Front. Microbiol. 2021, 12, 647242. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.L.; Li, Q.Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.M.; Hu, F.L. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
- Xin, P.; Xing, H.; Chu, W.H. Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microb. Pathog. 2022, 165, 105387. [Google Scholar] [CrossRef]
- Cornuault, J.K.; Byatt, G.; Paquet, M.E.; Koninck, P.D.; Moineau, S. Zebrafish: A big fish in the study of the gut microbiota. Curr. Opin. Biotechnol. 2022, 73, 308–313. [Google Scholar] [CrossRef]
- Ekmekciu, I.; Klitzing, E.V.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kühl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Lan, C.C.; Love, D.R. Molecular Characterisation of Bacterial Community Structure along the Intestinal Tract of Zebrafish (Danio rerio): A Pilot Study. ISRN Microbiol. 2012, 2012, 590385. [Google Scholar] [CrossRef] [Green Version]
- Adrià López, N.; Wakako, I.O.; Detmer Sipkema, D.P.; Charles, M.; Maria Forlenza, G.F.W.; Sylvia, B. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mahowald, M.A.; Goodman, A.L.; Trent, C.M.; Gordon, J.I. In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc. Natl. Acad. Sci. USA 2007, 104, 7622–7627. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.L.; Zhang, J.H. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, V.; Ravi, J.; Lauren, N.B.; Marwan, G.; Lisa, K.; James, E.K.; Romil, S.; David, A.; Matthew, S.J.; Naga, C. Oxidative Stress in Chronic Liver Disease: Relationship Between Peripheral and Hepatic Measurements. Am. J. Med. Sci. 2011, 34, 314–317. [Google Scholar] [CrossRef] [Green Version]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 9–57. [Google Scholar] [CrossRef] [PubMed]
- Khaper, N.; Bryan, S.; Dhingra, S.; Singal, R.; Bajaj, A.; Pathak, C.M.; Singal, P.K. Targeting the Vicious Inflammation-Oxidative Stress Cycle for the Management of Heart Failure. Antioxid. Redox Signal. 2010, 13, 1033–1049. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Singh, N.K.; Shukla, P.; Kirti, P.B. A CBL-interacting protein kinase AdCIPK5 confers salt and osmotic stress tolerance in transgenic tobacco. Sci. Rep. 2020, 10, 418. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Luo, H.; Vanek, K.N.; LaRue, A.C.; Schulte, B.A.; Wang, G.Y. Catalase inhibits ionizing radiation-induced apoptosis in hematopoietic stem and progenitor cells. Stem. Cells Dev. 2015, 24, 1342–1351. [Google Scholar] [CrossRef] [Green Version]
- Quintaneiro, C.; Teixeira, B.; Benede, J.L.; Chisvert, A.; Soares, A.; Monteiro, M.S. Toxicity effects of the organic UV-filter 4-Methylbenzylidene camphor in zebrafish embryos. Chemosphere 2019, 218, 273–281. [Google Scholar] [CrossRef]
- Huang, Y.X.; Li, H.F.; Huang, Z.B. Recent progress on antioxidant products from natural sources. J. Guangdong Pharm. Univ. 2016, 32, 532–536. [Google Scholar] [CrossRef]
- Zhou, S.Z.; Huang, G.L.; Chen, G.Y. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Z.; Xu, Z.J.; Liu, G.T.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef]
- Li, W.D.; Jiang, N.F.; Li, B.X.; Wan, M.H.; Chang, X.T.; Liu, H.; Zhang, L.; Yin, S.P.; Qi, H.M.; Liu, S.M. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef]
- da Silva Dantas Viegas, C.C.; Silva, A.S.; Braga, R.M.; de Andrade, H.H.N.; de Sousa Santos, A.K.F.; Leite Ferreira, M.D.; Ribeiro, M.D.; Silva, L.H.A.C.; de Lima, L.A.; Vanderlei de Souza, M.D.F.; et al. Antinociceptive, anti-inflammatory and antioxidant activities of the crude ethanolic extract and alkaloid fraction of Waltheria viscosissima A. St.-Hil. (Malvaceae). J. Ethnopharmacol. 2022, 292, 115173. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.X.; Jiang, H.X. A review on the structure-function relationship aspect of polysaccharides from tea materials. Crit. Rev. Food Sci. Nutr. 2015, 5, 930–938. [Google Scholar] [CrossRef]
- Chen, M.R.; Wang, S.Y.; Liang, X.; Ma, D.H.; He, L.; Liu, Y.W. Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan Supplementation on Serum Immune Parameters and Intestinal Immune-Related Gene Expression of Schizothorax prenanti. Int. J. Mol. Sci. 2017, 18, 2558. [Google Scholar] [CrossRef] [Green Version]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 9, 4240–4246. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Butkowski, E.G.; Jelinek, H.F. Hyperglycaemia, oxidative stress and inflammatory markers. Redox Rep. 2017, 22, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.Q.; Zhang, J.; Xiang, J.; Li, Y.; Wu, D.; Xu, J.J. Calcitriol inhibits ROS-NLRP3-IL-1beta signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019, 21, 101093. [Google Scholar] [CrossRef]
- Du, S.Q.; Wang, X.R.; Zhu, W.; Ye, Y.; Yang, J.W.; Ma, S.M.; Ji, C.S.; Liu, C.Z. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci. Ther. 2018, 24, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segev-Amzaleg, N.; Trudler, D.; Frenkel, D. Preconditioning to mild oxidative stress mediates astroglial neuroprotection in an IL-10-dependent manner. Brain Behav. Immun. 2013, 30, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gholamnezhad, Z.; Safarian, B.; Esparham, A.; Mirzaei, M.; Esmaeilzadeh, M.; Boskabady, M.H. The modulatory effects of exercise on lipopolysaccharide-induced lung inflammation and injury: A systemic review. Life Sci. 2022, 293, 120306. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mahowald, M.A.; Ley, R.E.; Gordon, J.I. Reciprocal Gut Microbiota Transplants from Zebrafish and Mice to Germ-free Recipients Reveal Host Habitat Selection. Cell 2006, 7, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.P.; Zhang, J.H.; Liu, X.Y.; Gan, L.; Xie, Y.; Zhang, H.; Si, J. The Function and the Affecting Factors of the Zebrafish Gut Microbiota. Front. Microbiol. 2022, 13, 903471. [Google Scholar] [CrossRef]
- Liu, S.R. The Development of Our Organ of Other Kinds—The Gut Microbiota. Front. Microbiol. 2016, 7, 2107. [Google Scholar] [CrossRef]
- Xu, K.H.; Zhang, Y.D.; Huang, Y.M.; Wang, J. Toxicological effects of microplastics and phenanthrene to zebrafish (Danio rerio). Sci. Total Environ. 2021, 757, 143730. [Google Scholar] [CrossRef]
- Wang, X.Y.; Shen, M.L.; Zhou, J.J.; Jin, Y.X. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 216, 19–28. [Google Scholar] [CrossRef]
- Ding, Q.W.; Hao, Q.; Zhang, Q.S.; Yang, Y.L.; Olsen, R.E.; Ringo, E.; Ran, C.; Zhang, Z.; Zhou, Z.G. Excess DHA Induces Liver Injury via Lipid Peroxidation and Gut Microbiota-Derived Lipopolysaccharide in Zebrafish. Front. Nutr. 2022, 9, 870343. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.X.; Xie, Y.D.; Li, Y.; Zhou, W.; Zhang, Z.; Yang, Y.L.; Olsen, R.E.; Ringo, E.; Ran, C.; Zhou, Z. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish Shellfish Immunol. 2022, 120, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.F.; Bi, W.W.; Chen, S.; Zhu, J.L.; Liu, X.X. Regulatory function of sigma factors RpoS/RpoN in adaptation and spoilage potential of Shewanella baltica. Food Microbiol. 2021, 97, 103755. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.C.; Wei, H.H.; Tian, C.Y.; Damron, F.H.; Zhou, J.Z.; Qiu, D.R. An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis. BMC Microbiol. 2015, 15, 34. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, O.N.; Mejean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef]
- Willms, R.J.; Jones, L.O.; Hocking, J.F.; Foley, E. A cell atlas of microbe-responsive processes in the zebrafish intestine. Cell Rep. 2022, 38, 110311. [Google Scholar] [CrossRef]
- Taormina, M.J.; Hay, E.A.; Parthasarathy, R. Passive and Active Microrheology of the Intestinal Fluid of the Larval Zebrafish. Biophys. Soc. 2017, 113, 957–965. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhang, Z.; Ding, Q.W.; Yang, Y.L.; Bindelle, J.; Ran, C.; Zhou, Z.G. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Wang, A.R.; Meng, D.L.; Hao, Q.; Xia, R.; Zhang, Q.S.; Ran, C.; Yang, Y.Y.L.; Li, D.J.; Liu, W.S.; Zhang, Z.; et al. Effect of supplementation of solid-state fermentation product of Bacillus subtilis HGcc-1 to high-fat diet on growth, hepatic lipid metabolism, epidermal mucus, gut and liver health and gut microbiota of zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.; Sha, Z.; Chen, C.M.; Yu, C.T.; Lv, N.Y.; Ji, P.; Wu, X.H.; Ma, T.H.; Cheng, H.B.; et al. Rosmarinic acid prevents refractory bacterial pneumonia through regulating Keap1/Nrf2-mediated autophagic pathway and mitochondrial oxidative stress. Free Radic. Biol. Med. 2021, 168, 247–257. [Google Scholar] [CrossRef]
- Wang, H.; Qi, S.Z.; Mu, X.Y.; Yuan, L.L.; Li, Y.R.; Qiu, J. Bisphenol F induces liver-gut alteration in zebrafish. Sci. Total. Environ. 2022, 851, 157974. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.H.; Cheng, H.D.; Duan, X.Y.; Zhang, H.H.; Wang, Y.D.; Gong, Z.Y.; Zhang, H.J.; Sun, H.W.; Wang, L. Diet preference of zebrafish (Danio rerio) for bio-based polylactic acid microplastics and induced intestinal damage and microbiota dysbiosis. J. Hazard. Mater. 2022, 429, 128332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wan, X.Z.; Wu, D.S.; Ouyang, Y.Z.; Gao, L.Y.; Chen, Z.X.; El-Seedi, H.R.; Wang, M.F.; Chen, X.H.; Zhao, C. Characterization of the structure and analysis of the anti-oxidant effect of microalga Spirulina platensis polysaccharide on Caenorhabditis elegans mediated by modulating microRNAs and gut microbiota. Int. J. Biol. Macromol. 2020, 163, 2295–2305. [Google Scholar] [CrossRef] [PubMed]







| Group | 0.9% Normal Saline | 50% Ethanol | 0.2% Oxazolone | 0.3% SBE | Quantity per Group |
|---|---|---|---|---|---|
| CON | + | − | − | − | 30 |
| EtOH | − | + | − | − | 30 |
| Oxa | − | + | + | − | 30 |
| SBE | − | + | + | + | 30 |
| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| IL-10 | F | TGCGGGCAATATGAAGTC |
| R | TTCGCCATGAGCATGTCC | |
| IL-1β | F | AGGGCTTTCCTTTAAGACTG |
| R | ATATCCCGCTTGAGTTCC | |
| TNF-α | F | GGCGCTTTTGGATGTT |
| R | TTGCCCTGGGTCTTATG | |
| β-actin | F | ACCGCTGCCTCTTCTT |
| R | GCAATGCCAGGGTACA |
| Main Active Substances | Contents (%) |
|---|---|
| Polysaccharides | 50.14% |
| Alkaloids | 20.03% |
| Polyphenols | 7.27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhou, W.-W.; Shi, D.-D.; Pan, F.-F.; Sun, W.-W.; Yang, P.-L.; Li, X.-M. The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish. Antioxidants 2023, 12, 192. https://doi.org/10.3390/antiox12010192
Yang J, Zhou W-W, Shi D-D, Pan F-F, Sun W-W, Yang P-L, Li X-M. The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish. Antioxidants. 2023; 12(1):192. https://doi.org/10.3390/antiox12010192
Chicago/Turabian StyleYang, Juan, Wei-Wei Zhou, Dong-Dong Shi, Fang-Fang Pan, Wen-Wen Sun, Pei-Long Yang, and Xiu-Mei Li. 2023. "The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish" Antioxidants 12, no. 1: 192. https://doi.org/10.3390/antiox12010192
APA StyleYang, J., Zhou, W. -W., Shi, D. -D., Pan, F. -F., Sun, W. -W., Yang, P. -L., & Li, X. -M. (2023). The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish. Antioxidants, 12(1), 192. https://doi.org/10.3390/antiox12010192