The Common Single Cause of Chronic Multi-Hormonal Resistance in Oxidative Stress
Abstract
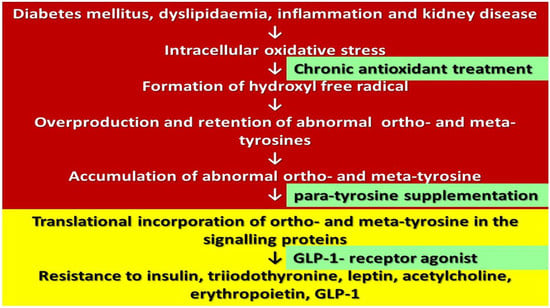
:1. Introduction
2. Intracellular Signaling of Insulin, Leptin, Acetylcholine, Glucagone-Like Peptide-1, Erythropoietin and Triiodothyronine
2.1. Insulin Signaling
2.2. Triiodothyronine Signaling
2.3. Leptin Signaling
2.4. Acetylcholine Signaling
2.5. Erythropoietin Signaling
2.6. Glucagone-Like Peptide-1 (GLP-1) Signaling
2.7. Common and Distinct Signaling Pathways
2.8. Acute Redox Regulation of Hormonal Signaling and Metabolism
2.9. The Oxidative Stress Memory
2.10. The Breakthrough Phenomenon
2.11. Consequences of Oxidative Stress at the Protein and Amino Acid Level
2.12. Sources of Normal (Para) and Abnormal (Meta and Ortho) Tyrosines
2.13. Overproduction and Reduced Elimination of Abnormal Tyrosines
2.14. Translational Incorporation of Abnormal Tyrosines into the Proteins
3. Abnormal Tyrosine Content of Proteins as a Common Cause of Chronic Multi-Hormonal Resistance
3.1. Insulin and GLP-1 Resistance
3.2. Triiodothyronine Resistance
3.3. Acetylcholine Resistance
3.4. Erythropoietin Resistance
3.5. Effect of Antioxidants on Multi-Hormonal Resistance
4. Conclusions
5. Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Mastrototaro, L.; Roden, M. Insulin resistance and insulin sensitizing agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Moon, M.J.; Kim, H.Y.; Park, S.; Kim, D.; Cho, E.B.; Hwang, J.; Seong, J.Y. Insulin contributes to fine-tuning of the pancreatic beta-cell response to glucagon-like peptide-1. Mol. Cells 2011, 32, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, M.G.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic abnormalities and oxidative stress in lupus. Curr. Opin. Rheumatol. 2017, 29, 442–449. [Google Scholar] [CrossRef]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.N.; McGillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.R. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Carlson, G.L. Hunterian Lecture: Insulin resistance in human sepsis: Implications for the nutritional and metabolic care of the critically ill surgical patient. Ann. R Coll. Surg. Engl. 2004, 86, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br. J. Pharmacol. 2011, 162, 597–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frühbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393 (Pt 1), 7–20. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.C.; Lord, R.A.; Anderson, G.M. Multiple leptin signalling pathways in the control of metabolism and fertility: A means to different ends? Int. J. Mol. Sci. 2021, 22, 9210. [Google Scholar] [CrossRef]
- Münzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, H.G.; Priviero, F.B.M.; Souza, C.T.; Zecchin, K.G.; Prada, P.O.; Carvalheira, J.B.C.; Velloso, L.A.; Antunes, E.; Saad, M.J.A. Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes 2007, 56, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Watowich, S.S. The Erythropoietin Receptor: Molecular structure and hematopoietic signaling pathways. J. Investig. Med. 2011, 59, 1067–1072. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, X.H.; Guo, L.L.; Gu, Y.H.; Qiao, Q.Y.; Jin, H.M. Erythropoietin reduces insulin resistance via regulation of its receptor-mediated signaling pathways in db/db mice skeletal muscle. Int. J. Biol. Sci. 2017, 13, 1329–1340. [Google Scholar] [CrossRef] [Green Version]
- Holz, G.G.; Chepurny, O.G. Diabetes outfoxed by GLP-1? Sci. STKE 2005, 2005, pe2. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.; Yin, M.; Jin, Z.G. Essential roles of Gab1 tyrosine phosphorylation in growth factor-mediated signaling and angiogenesis. Int. J. Cardiol. 2015, 181, 180–184. [Google Scholar] [CrossRef]
- Mohás-Cseh, J.; Molnár, G.A.; Pap, M.; Laczy, B.; Vas, T.; Kertész, M.; Németh, K.; Hetényi Cs Csikós, O.; Tóth, G.K.; Reményi, A.; et al. Incorporation of oxidized phenylalanine derivatives into insulin signaling relevant proteins may link oxidative stress to signaling conditions underlying chronic insulin resistance. Biomedicines 2022, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free. Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Jermendy, G.Y. Vascular memory: Can we broaden the concept of the metabolic memory? Cardiovasc. Diabetol. 2012, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Garvey, W.T.; Olefsky, J.M.; Griffin, J.; Hamman, R.F.; Kolterman, O.G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 1985, 34, 222–234. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Xu, W.; Shi, L.; Zhang, Q.; Zhu, D.; Hu, Y.; Zhou, Z.; Yan, X.; Tian, H.; et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet 2008, 371, 1753–1760. [Google Scholar] [CrossRef]
- Højberg, P.V.; Vilsbøll, T.; Rabøl, R.; Knop, F.K.; Bache, M.; Krarup, T.; Holst, J.J.; Madsbad, S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009, 52, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.M.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 receptor agonist tirzepatide improves Beta-cell function and insulin sensitivity in type 2 diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, G.A.; Wagner, Z.; Markó, L.; Kőszegi, T.; Mohás, M.; Kocsis, B.; Matus, Z.; Wagner, L.; Tamaskó, M.; Mazák, I.; et al. Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: Evidence for hydroxyl radical production. Kidney Int. 2005, 68, 2281–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szijártó, I.A.; Molnár, G.A.; Mikolás, E.; Fisi, V.; Cseh, J.; Laczy, B.; Kovács, T.; Böddi, K.; Takátsy, A.; Gollasch, M.; et al. Elevated vascular level of ortho-tyrosine contributes to the impairment of insulin-induced arterial relaxation. Horm. Metab. Res. 2014, 46, 749–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sélley, E.; Kun Sz Kürthy, M.; Kovács, T.; Wittmann, I.; Molnár, G.A. Para-tyrosine supplementation improves insulin- and liraglutide-induced vasorelaxation in cholesterol-fed rats. Protein. Pept. Lett. 2015, 22, 736–742. [Google Scholar] [CrossRef]
- Szélig, L.; Kun Sz Woth, G.; Molnár, G.A.; Zrínyi, Z.; Kátai, E.; Lantos, J.; Wittmann, I.; Bogár, L.; Miseta, A.; Csontos, C.S. Time courses of changes of para-, meta-, and ortho-tyrosine in septic patients: A pilot study. Redox Rep. 2016, 21, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Kun, S.Z.; Mikolás, E.; Molnár, G.A.; Sélley, E.; Laczy, B.; Csiky, B.; Kovács, T.; Wittmann, I. Association of plasma ortho-tyrosine/para-tyrosine ratio with responsiveness of erythropoiesis-stimulating agent in dialyzed patients. Redox Rep. 2014, 19, 190–198. [Google Scholar] [CrossRef]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137 (Suppl. S1), 1586S–1590S; discussion 1597S–1598S. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.J.; Wang, H.; Fu, S.; Dean, R.T. Biosynthetic incorporation of oxidized amino acids into proteins and their cellular proteolysis. Free Radic. Biol. Med. 2002, 32, 766–775. [Google Scholar] [CrossRef]
- Ozawa, K.; Headlam, M.J.; Mouradov, D.; Watt, S.J.; Beck, J.L.; Rodgers, K.J.; Dean, R.T.; Huber, T.; Otting, G.; Dixon, N.E. Translational incorporation of L-3,4-dihydroxyphenylalanine into proteins. FEBS J. 2005, 272, 3162–3171. [Google Scholar] [CrossRef]
- Wang, Z.; Matthews, H. Translational incorporation of modified phenylalanines and tyrosines during cell-free protein synthesis. RSC Adv. 2020, 10, 11013–11023. [Google Scholar] [CrossRef]
- Zer, H.; Mizrahi, H.; Malchenko, N.; Avin-Wittenberg, T.; Klipcan, L.; Ostersetzer-Biran, O. The Phytotoxicity of meta-tyrosine is associated with altered phenylalanine metabolism and misincorporation of this non-proteinogenic Phe-analog to the Plant’s Proteome. Front. Plant Sci. 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Mikolás, E.; Kun Sz Laczy, B.; Molnár, M.G.; Sélley, E.; Kőszegi, T.; Wittmann, I. Incorporation of ortho- and meta-tyrosine into cellular proteins leads to erythropoietin-resistance in an erythroid cell line. Kidney Blood Press. Res. 2013, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Kun, S.Z.; Molnár, G.A.; Sélley, E.; Szélig, L.; Bogár, L.; Csontos, C.S.; Miseta, A. Wittmann 1. Insulin therapy of nondiabetic septic patients is predicted by para-tyrosine/phenylalanine ratio and by hydroxyl radical-derived products of phenylalanine. Oxid. Med. Cell Longev. 2015, 2015, 839748. [Google Scholar] [CrossRef] [Green Version]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Kertész, M.; Kun Sz Sélley, E.; Nagy Zs Kőszegi, T.; Wittmann, I. A breakthrough-like effect of metformin reduces peripheral resistance to triiodothyronine in euthyroid, non-insulin-resistant, type 2 diabetic patients. Endocr. Connect. 2021, 10, 782–788. [Google Scholar] [CrossRef]
- Szijártó, I.A.; Molnár, G.A.; Mikolás, E.; Fisi, V.; Laczy, B.; Gollasch, M.; Koller, A.; Wittmann, I. Increase in insulin-induced relaxation of consecutive arterial segments toward the periphery: Role of vascular oxidative state. Free Radic. Res. 2014, 48, 749–757. [Google Scholar] [CrossRef]
- Molnár, G.A.; Mikolás, E.; Szijártó, I.A.; Kun Sz Sélley, E.; Wittmann, I. Tyrosine isomers and hormonal signaling: A possible role for the hydroxyl free radical in insulin resistance. World J. Diabetes 2015, 6, 500–507. [Google Scholar] [CrossRef]
- Rossert, J.; Gassmann-Mayer, C.; Frei, D.; McClellan, W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol. Dial. Transplant. 2007, 22, 794–800. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. A clinical trial about a food aupplement containing α-Lipoic acid on oxidative stress markers in type 2 diabetic patients. Int. J. Mol. Sci. 2016, 17, 1802. [Google Scholar] [CrossRef]
- Capasso, I.; Esposito, E.; Maurea, N.; Montella, M.; Crispo, M.; De Laurentiis, M.; D’Aiuto, M.; Frasci, G.; Botti, G.; Grimaldi, M.; et al. Combination of inositol and alpha lipoic acid in metabolic syndrome-affected women: A randomized placebo-controlled trial. Trials 2013, 14, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Han, P.; Wu, N.; He, B.; Lu, Y.; Li, S.; Liu, S.; Zhao, S.; Liu, L.; Li, L. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity. (Silver Spring) 2011, 19, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, M.V. Influence of age and dose on the effect of resveratrol for glycemic control in type 2 diabetes mellitus: Systematic review and meta-analysis. Molecules 2022, 27, 5232. [Google Scholar] [CrossRef] [PubMed]
- Hamid, D.Z.A.; Nienaa, Y.A.; Mostafa, T.M. Alpha-lipoic acid improved anemia, erythropoietin resistance, maintained glycemic control, and reduced cardiovascular risk in diabetic patients on hemodialysis: A multi-center prospective randomized controlled study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, G.; Baggetta, R.; Tripepi, G.; Galli, F.; Bolignano, D. Effects of vitamin E-coated versus conventional membranes in chronic hemodialysis patients: A systematic review and meta-analysis. Blood Purif. 2017, 43, 101–122. [Google Scholar] [CrossRef]
- Lyu, X.; Yan, K.; Wang, X.; Xu, H.; Guo, X.; Zhu, H.; Pan, H.; Wang, L.; Yang, H.; Gong, F. A novel anti-obesity mechanism for liraglutide by improving adipose tissue leptin resistance in high-fat diet-fed obese mice. Endocr. J. 2022, 69, 1233–1244. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, P.; Hong, T.; Tang, S.; Meng, R.; Bi, Y.; Zhu, D. Erythropoietin alleviates hepatic insulin resistance via PPARγ-dependent AKT activation. Sci. Rep. 2015, 5, 17878. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; LEADER Steering Committee; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; REWIND Investigators; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino RBSr Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; Harmony Outcomes committees and investigators; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; SUSTAIN-6 Investigators; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; PIONEER 6 Investigators; et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef] [PubMed]



| Sources of Para-Tyrosine | Sources of Meta- and Ortho-Tyrosine |
|---|---|
| Food Enzymatic formation Hydroxyl free radical | Food? Enzymatic formation(in plants) Hydroxyl free radical |
| Overproduction of Abnormal Tyrosines | Reduced Elimination of Abnormal Tyrosines |
|---|---|
| Inflammation Systemic subclinical e.g., Diabetes mellitus Dyslipidaemia Clinically overt e.g., Sepsis | Chronic kidney disease End-stage kidney disease Dialysis therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittmann, I. The Common Single Cause of Chronic Multi-Hormonal Resistance in Oxidative Stress. Antioxidants 2023, 12, 75. https://doi.org/10.3390/antiox12010075
Wittmann I. The Common Single Cause of Chronic Multi-Hormonal Resistance in Oxidative Stress. Antioxidants. 2023; 12(1):75. https://doi.org/10.3390/antiox12010075
Chicago/Turabian StyleWittmann, István. 2023. "The Common Single Cause of Chronic Multi-Hormonal Resistance in Oxidative Stress" Antioxidants 12, no. 1: 75. https://doi.org/10.3390/antiox12010075
APA StyleWittmann, I. (2023). The Common Single Cause of Chronic Multi-Hormonal Resistance in Oxidative Stress. Antioxidants, 12(1), 75. https://doi.org/10.3390/antiox12010075