Serum Selenium-Binding Protein 1 (SELENBP1) in Burn Injury: A Potential Biomarker of Disease Severity and Clinical Course
Abstract
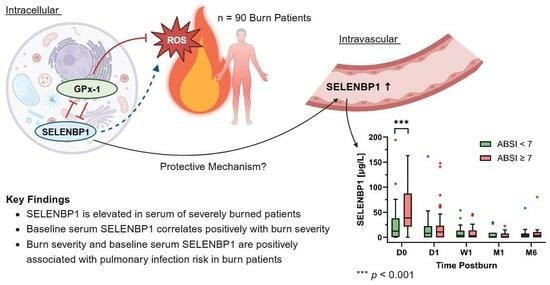
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling and Measurements
2.3. Statistical Analyses
3. Results
3.1. Patient Demographic and Clinical Characteristics
3.2. SELENBP1 Is Elevated following a Severe Burn
3.3. Baseline SELENBP1 Is Positively Associated with Burn Severity
3.4. Correlation Analysis of SELENBP1
3.5. Baseline SELENBP1 and Burn Severity Are Associated with the Risk of Pulmonary Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behne, D.; Kyriakopoulos, A. Mammalian selenium-containing proteins. Annu. Rev. Nutr. 2001, 21, 453–473. [Google Scholar] [CrossRef]
- Bansal, M.P.; Oborn, C.J.; Danielson, K.G.; Medina, D. Evidence for two selenium-binding proteins distinct from glutathione peroxidase in mouse liver. Carcinogenesis 1989, 10, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Elhodaky, M.; Diamond, A.M. Selenium-Binding Protein 1 in Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, W.; Li, M.; Byun, D.S.; Tong, C.; Nasser, S.; Zhuang, M.; Arango, D.; Mariadason, J.M.; Augenlicht, L.H. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Micoogullari, M.; Hoang, N.A.; Bergheim, I.; Klotz, L.O.; Sies, H. Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol. 2019, 20, 489–495. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Wang, Y.; Sytkowski, A.J. Human selenium binding protein-1 (hSP56) interacts with VDU1 in a selenium-dependent manner. Biochem. Biophys. Res. Commun. 2009, 379, 583–588. [Google Scholar] [CrossRef]
- Porat, A.; Sagiv, Y.; Elazar, Z. A 56-kDa selenium-binding protein participates in intra-Golgi protein transport. J. Biol. Chem. 2000, 275, 14457–14465. [Google Scholar] [CrossRef]
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.O. Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase. Redox Biol. 2023, 65, 102807. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.P.; Ip, C.; Medina, D. Levels and 75Se-labeling of specific proteins as a consequence of dietary selenium concentration in mice and rats. Proc. Soc. Exp. Biol. Med. 1991, 196, 147–154. [Google Scholar] [CrossRef]
- Diamond, A.M. The subcellular location of selenoproteins and the impact on their function. Nutrients 2015, 7, 3938–3948. [Google Scholar] [CrossRef]
- Fang, W.; Goldberg, M.L.; Pohl, N.M.; Bi, X.; Tong, C.; Xiong, B.; Koh, T.J.; Diamond, A.M.; Yang, W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 2010, 31, 1360–1366. [Google Scholar] [CrossRef]
- Huang, C.; Ding, G.; Gu, C.; Zhou, J.; Kuang, M.; Ji, Y.; He, Y.; Kondo, T.; Fan, J. Decreased selenium-binding protein 1 enhances glutathione peroxidase 1 activity and downregulates HIF-1alpha to promote hepatocellular carcinoma invasiveness. Clin. Cancer Res. 2012, 18, 3042–3053. [Google Scholar] [CrossRef]
- Udawela, M.; Money, T.T.; Neo, J.; Seo, M.S.; Scarr, E.; Dean, B.; Everall, I.P. SELENBP1 expression in the prefrontal cortex of subjects with schizophrenia. Transl. Psychiatry 2015, 5, e615. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.W.; Bui, M.A.T.; Kim, Y.; Kim, M.; Park, J.C.; Kim, N.H.; Pyeon, G.H.; Jo, Y.S.; Jang, J.; et al. SELENBP1 overexpression in the prefrontal cortex underlies negative symptoms of schizophrenia. Proc. Natl. Acad. Sci. USA 2022, 119, e2203711119. [Google Scholar] [CrossRef]
- Seelig, J.; Heller, R.A.; Haubruck, P.; Sun, Q.; Georg Klingenberg, J.; Hackler, J.; Crowell, H.L.; Daniel, V.; Moghaddam, A.; Schomburg, L.; et al. Selenium-Binding Protein 1 (SELENBP1) as Biomarker for Adverse Clinical Outcome after Traumatic Spinal Cord Injury. Front. Neurosci. 2021, 15, 680240. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.C.; Slagman, A.; Kuhn-Heid, E.C.D.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Mockel, M.; Schomburg, L. Circulating levels of selenium-binding protein 1 (SELENBP1) are associated with risk for major adverse cardiac events and death. J. Trace Elem. Med. Biol. 2019, 52, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Heid, E.C.D.; Kuhn, E.C.; Ney, J.; Wendt, S.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Schomburg, L. Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery. Nutrients 2019, 11, 2005. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef] [PubMed]
- Bertin-Maghit, M.; Goudable, J.; Dalmas, E.; Steghens, J.P.; Bouchard, C.; Gueugniaud, P.Y.; Petit, P.; Delafosse, B. Time course of oxidative stress after major burns. Intensive Care Med. 2000, 26, 800–803. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A. Trace elements in trauma and burns. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Eggimann, P.; Heyland, D.K.; Chiolero, R.L.; Revelly, J.P.; Day, A.; Raffoul, W.; Shenkin, A. Reduction of nosocomial pneumonia after major burns by trace element supplementation: Aggregation of two randomised trials. Crit. Care 2006, 10, R153. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Krishnan, P.; Frew, Q.; Green, A.; Martin, R.; Dziewulski, P. Cause of death and correlation with autopsy findings in burns patients. Burns 2013, 39, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef]
- Fitzwater, J.; Purdue, G.F.; Hunt, J.L.; O’Keefe, G.E. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J. Trauma. 2003, 54, 959–966. [Google Scholar] [CrossRef]
- Tobiasen, J.; Hiebert, J.M.; Edlich, R.F. The abbreviated burn severity index. Ann. Emerg. Med. 1982, 11, 260–262. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bengtsson, Y.; Demircan, K.; Vallon-Christersson, J.; Malmberg, M.; Saal, L.H.; Ryden, L.; Borg, A.; Schomburg, L.; Sandsveden, M.; Manjer, J. Serum copper, zinc and copper/zinc ratio in relation to survival after breast cancer diagnosis: A prospective multicenter cohort study. Redox Biol. 2023, 63, 102728. [Google Scholar] [CrossRef]
- Hybsier, S.; Schulz, T.; Wu, Z.; Demuth, I.; Minich, W.B.; Renko, K.; Rijntjes, E.; Kohrle, J.; Strasburger, C.J.; Steinhagen-Thiessen, E.; et al. Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated ELISA for selenoprotein P. Redox Biol. 2017, 11, 403–414. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Osborne, T.; Wall, B.; Edgar, D.W.; Fairchild, T.; Wood, F. Current understanding of the chronic stress response to burn injury from human studies. Burns Trauma 2023, 11, tkad007. [Google Scholar] [CrossRef] [PubMed]
- Osuka, A.; Ogura, H.; Ueyama, M.; Shimazu, T.; Lederer, J.A. Immune response to traumatic injury: Harmony and discordance of immune system homeostasis. Acute Med. Surg. 2014, 1, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.G.; Vlig, M.; Fasse, E.; Stoop, M.M.; Pijpe, A.; van Zuijlen, P.P.M.; Joosten, I.; Boekema, B.; Koenen, H. Burn-injured skin is marked by a prolonged local acute inflammatory response of innate immune cells and pro-inflammatory cytokines. Front. Immunol. 2022, 13, 1034420. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Herndon, D.N.; Przkora, R.; Pereira, C.T.; Oliveira, H.M.; Queiroz, D.M.; Rocha, A.M.; Jeschke, M.G. Cytokine expression profile over time in severely burned pediatric patients. Shock 2006, 26, 13–19. [Google Scholar] [CrossRef]
- Yamada, Y.; Endo, S.; Inada, K. Plasma cytokine levels in patients with severe burn injury--with reference to the relationship between infection and prognosis. Burns 1996, 22, 587–593. [Google Scholar] [CrossRef]
- Laggner, M.; Lingitz, M.T.; Copic, D.; Direder, M.; Klas, K.; Bormann, D.; Gugerell, A.; Moser, B.; Radtke, C.; Hacker, S.; et al. Severity of thermal burn injury is associated with systemic neutrophil activation. Sci. Rep. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ruthenborg, R.J.; Ban, J.J.; Wazir, A.; Takeda, N.; Kim, J.W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells 2014, 37, 637–643. [Google Scholar] [CrossRef]
- Bierl, C.; Voetsch, B.; Jin, R.C.; Handy, D.E.; Loscalzo, J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J. Biol. Chem. 2004, 279, 26839–26845. [Google Scholar] [CrossRef]
- Scortegagna, M.; Martin, R.J.; Kladney, R.D.; Neumann, R.G.; Arbeit, J.M. Hypoxia-inducible factor-1alpha suppresses squamous carcinogenic progression and epithelial-mesenchymal transition. Cancer Res. 2009, 69, 2638–2646. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Zhou, J.R.; Gao, C.; Feldman, L.; Sytkowski, A.J. Human selenium binding protein-1 (hSP56) is a negative regulator of HIF-1alpha and suppresses the malignant characteristics of prostate cancer cells. BMB Rep. 2014, 47, 411–416. [Google Scholar] [CrossRef]
- Forceville, X.; Mostert, V.; Pierantoni, A.; Vitoux, D.; Le Toumelin, P.; Plouvier, E.; Dehoux, M.; Thuillier, F.; Combes, A. Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur. Surg. Res. 2009, 43, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.B.; Crack, P.J.; Flentjar, N.; Iannello, R.C.; Hertzog, P.J.; Kola, I. An imbalance in antioxidant defense affects cellular function: The pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (Gpx1) knockout mouse. Redox Rep. 2003, 8, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zeng, H.; Wu, R.T.; Cheng, W.H. Loss of Selenium-Binding Protein 1 Decreases Sensitivity to Clastogens and Intracellular Selenium Content in HeLa Cells. PLoS ONE 2016, 11, e0158650. [Google Scholar] [CrossRef] [PubMed]



| Variables | Pulmonary Infection (n = 37) | Other Infections (n = 22) | No Infection (n = 31) | Total (n = 90) |
|---|---|---|---|---|
| Sex Female Male | 8 (21.6) 29 (78.4) | 6 (27.3) 16 (72.7) | 3 (9.7) 28 (90.3) | 17 (18.9) 73 (81.1) |
| Age (yr) | 51.4 ± 17.9 | 40.6 ± 17.4 | 42.4 ± 16.1 | 45.7 ± 17.7 |
| BMI (kg/m2) | 24.9 (22.5–28.4) | 27.2 (22.7–29.9) | 26.2 (22.8–30.8) | 25.7 (22.6–29.3) |
| TBSA (%) | 35.0 (26.0–40.0) | 30.0 (22.9–50.8) | 20.0 (13.5–29.5) | 29.0 (20.0–37.0) |
| Full-thickness burn | 26 (70.3) | 16 (72.7) | 10 (32.3) | 52 (57.8) |
| Inhalation injury | 15 (40.5) | 4 (18.2) | 3 (9.7) | 22 (24.4) |
| ABSI | 8.0 (7.0–10.0) | 7.0 (6.0–9.0) | 6.0 (4.0–7.0) | 7.0 (6.0–9.0) |
| SELENBP1 (µg/L) | 45.6 (21.2–70.5) | 35.8 (8.7–61.6) | 12.5 (3.7–34.2) | 26.9 (10.0–61.0) |
| Sepsis | 36 (97.3) | 19 (86.4) | 0 (0.0) | 55 (61.1) |
| Mortality | 3 (8.1) | 1 (4.5) | 1 (3.2) | 5 (5.6) |
| Length of stay (d) | 54.0 (21.0–87.0) | 31.5 (23.3–44.8) | 20.0 (10.0–25.0) | 27.0 (19.0–56.0) |
| Length of ICU stay (d) | 32.0 (17.0–54.0) | 18.5 (13.3–27.0) | 8.0 (2.0–14.0) | 17.5 (8.3–40.8) |
| SELENBP1 Correlations (p < 0.05) | |||||||
|---|---|---|---|---|---|---|---|
| D0 | D1 | D2 | D3 | W1 | W2 | Full | |
| Se | –0.282 | –0.227 | –0.230 | ||||
| SELENOP | –0.382 | –0.305 | –0.303 | –0.210 | |||
| GPx-3 | –0.170 | ||||||
| Cu | –0.127 | ||||||
| Zn | –0.124 | ||||||
| WBCs | 0.421 | 0.249 | –0.240 | 0.197 | |||
| CRP | 0.260 | –0.243 | |||||
| PCT | 0.340 | 0.253 | 0.391 | 0.228 | |||
| Cut-Off Point | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | |
|---|---|---|---|---|---|
| SELENBP1 | >21.2 µg/L | 75.7 (59.9–86.6) | 64.5 (47.0–78.9) | 59.8 | 79.2 |
| ABSI | >6.5 | 86.5 (72.0–94.1) | 71.0 (53.4–83.9) | 67.5 | 88.3 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Baseline SELENBP1 > 21.2 µg/L | 2.47 | 0.68–8.88 | 0.164 |
| ABSI > 6.5 | 14.37 | 3.15–88.78 | 0.001 |
| Age | 0.99 | 0.94–1.03 | 0.574 |
| Sex (Female) | 1.41 | 0.26–9.50 | 0.707 |
| Intercept | 0.25 | 0.03–1.66 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turan, T.L.; Klein, H.J.; Hackler, J.; Hoerner, L.; Rijntjes, E.; Graf, T.R.; Plock, J.A.; Schomburg, L. Serum Selenium-Binding Protein 1 (SELENBP1) in Burn Injury: A Potential Biomarker of Disease Severity and Clinical Course. Antioxidants 2023, 12, 1927. https://doi.org/10.3390/antiox12111927
Turan TL, Klein HJ, Hackler J, Hoerner L, Rijntjes E, Graf TR, Plock JA, Schomburg L. Serum Selenium-Binding Protein 1 (SELENBP1) in Burn Injury: A Potential Biomarker of Disease Severity and Clinical Course. Antioxidants. 2023; 12(11):1927. https://doi.org/10.3390/antiox12111927
Chicago/Turabian StyleTuran, Tabael L., Holger J. Klein, Julian Hackler, Livia Hoerner, Eddy Rijntjes, Theresia Reding Graf, Jan A. Plock, and Lutz Schomburg. 2023. "Serum Selenium-Binding Protein 1 (SELENBP1) in Burn Injury: A Potential Biomarker of Disease Severity and Clinical Course" Antioxidants 12, no. 11: 1927. https://doi.org/10.3390/antiox12111927
APA StyleTuran, T. L., Klein, H. J., Hackler, J., Hoerner, L., Rijntjes, E., Graf, T. R., Plock, J. A., & Schomburg, L. (2023). Serum Selenium-Binding Protein 1 (SELENBP1) in Burn Injury: A Potential Biomarker of Disease Severity and Clinical Course. Antioxidants, 12(11), 1927. https://doi.org/10.3390/antiox12111927