Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract
Abstract
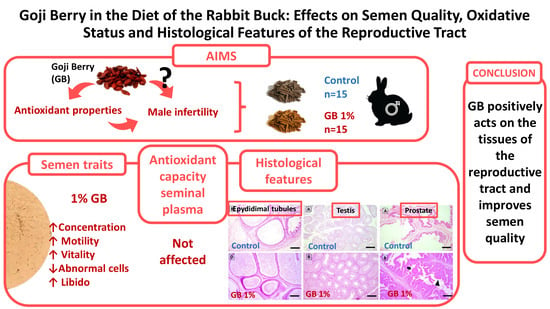
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sampling of Semen and Reproductive Organs
2.3. Seminal Quality Assessment
- Volume (mL): determined by graduated tubes;
- Color: defined as milky (normal), cream, yellowish, or transparent;
- Presence of gel (yes or no);
- Concentration (106 spermatozoa/mL): measured with the direct cell count method using a Burker chamber with a 40× objective after dilution of the semen 1:100;
- Motility (%): evaluated by placing two drops of fresh semen on a warm microscope slide and covering it with a glass cover slip. The percentage of motility was calculated after the evaluation of at least 10 microscopic fields with 100× magnification and 10 microscopic fields with 400× magnification;
- Live spermatozoa (%): determined using an eosin–nigrosin blue staining mixture and counting 200 cells. Specifically, 10 µL of undiluted semen was combined with 10 µL of eosin–nigrosin in a 1 mL Eppendorf tube (Eppendorf, Hamburg, Germany), gently mixed, and placed on a microscopic slide for evaluation. Cells that excluded the eosin stain, appearing white, were classified as live cells, while those with compromised or damaged membranes, colored with eosin and appearing pink, were considered dead;
- Abnormal spermatozoa (%): determined using an eosin–nigrosin blue staining mixture and counting 200 cells. Abnormalities were evaluated in all cases, focusing on the abnormal head and tail defects.
2.4. Oxidative and Inflammatory Status of Seminal Plasma
2.5. Histological Analysis
2.6. Statistical Analyses
3. Results
3.1. Clinical Evaluation and Body Weight
3.2. Semen Quality Assessment
3.3. Antioxidant and Inflammatory Parameters in Seminal Plasma
3.4. Histological Analysis
3.5. Correlations between Seminal Parameters, TAC, Antioxidant Enzymes, and Interleukin-1β
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.; Zhou, Z.W.; Sheng, H.P.; He, L.J.; Fan, X.W.; He, Z.X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An Evidence-Based Update on the Pharmacological Activities and Possible Molecular Targets of Lycium barbarum Polysaccharides. Drug Des. Devel. Ther. 2015, 9, 33–78. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Yao, H.; Gao, H.; Zhou, X.; Ma, X.; Xu, C.; Chen, J.; Han, J.; Pang, X.; Xu, R.; et al. Super Food Lycium barbarum (Solanaceae) Traceability via an Internal Transcribed Spacer 2 Barcode. Food Res. Int. 2013, 54, 1699–1704. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–792. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. Effect of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells. J. Biotechnol. 2016, 231, S26–S27. [Google Scholar] [CrossRef]
- Bo, R.; Sun, Y.; Zhou, S.; Ou, N.; Gu, P.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Simple Nanoliposomes Encapsulating Lycium barbarum Polysaccharides as Adjuvants Improve Humoral and Cellular Immunity in Mice. Int. J. Nanomed. 2017, 12, 6289–6301. [Google Scholar] [CrossRef]
- Lian, Y.Z.; Lin, I.H.; Yang, Y.C.; Chao, J.C.J. Gastroprotective Effect of Lycium barbarum Polysaccharides and C-Phyocyanin in Rats with Ethanol-Induced Gastric Ulcer. Int. J. Biol. Macromol. 2020, 165, 1519–1528. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.; Lo, A.C.Y.; Chan, T.C.Y.; So, K.F.; Lai, J.S.M.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium barbarum Polysaccharides in Disease. Biomed. Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxid. Med. Cell Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A Review of Botanical Characteristics, Phytochemistry, Clinical Relevance in Efficacy and Safety of Lycium barbarum Fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; de Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and Antioxidant Activity of Proteins from Feijoa sellowiana Berg. Fruit before and after in Vitro Gastrointestinal Digestion. Nat. Prod. Res. 2020, 34, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2021, 8, 799294. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens. Health 2014, 32, 1088727. [Google Scholar] [CrossRef]
- Zorgniotti, A.W.; Sealfon, A.I.; Toth, A. Further Clinical Experience with Testis Hypothermia for Infertility Due to Poor Semen. Urology 1982, 19, 636–640. [Google Scholar] [CrossRef]
- Lancranjan, I.; Măicănesgu, M.; Rafailaj, E.; Klepsgh, I.; Popesgu, H.I. Gonadic Function in Workmen with Long-Term Exposure to Microwaves. Health Phys. 1975, 29, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S. The Roles of Omega-3 and Omega-6 Fatty Acids in Idiopathic Male Infertility. Asian J. Androl. 2012, 14, 514–515. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Aitken, R.J. The Role of Sperm Oxidative Stress in Male Infertility and the Significance of Oral Antioxidant Therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary N-3 Source on Rabbit Male Reproduction. Oxid. Med. Cell Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef]
- De Gennaro, L.; Balistreri, S.; Lenzi, A.; Lombardo, F.; Ferrara, M.; Gandini, L. Psychosocial Factors Discriminate Oligozoospermic from Normozoospermic Men. Fertil. Steril. 2003, 79, 1571–1576. [Google Scholar] [CrossRef]
- Glazer, C.H.; Bonde, J.P.; Eisenberg, M.L.; Giwercman, A.; Hærvig, K.K.; Rimborg, S.; Vassard, D.; Pinborg, A.; Schmidt, L.; Bräuner, E.V. Male Infertility and Risk of Nonmalignant Chronic Diseases: A Systematic Review of the Epidemiological Evidence. Semin. Reprod. Med. 2017, 35, 282–290. [Google Scholar] [CrossRef]
- Whorton, D.; Krauss, R.M.; Marshall, S.; Milby, T.H. Infertility in Male Pesticide Workers. Lancet 1977, 310, 1259–1261. [Google Scholar] [CrossRef]
- Selevan, S.G.; Borkovec, L.; Slott, V.L.; Zudová, Z.; Rubeš, J.; Evenson, D.P.; Perreault, S.D. Semen Quality and Reproductive Health of Young Czech Men Exposed to Seasonal Air Pollution. Environ. Health Perspect. 2000, 108, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Retterstøl, K.; Hauger, T.B.; Tran, T.N.; Christophersen, B.O. Studies on the Metabolism of Essential Fatty Acids in Isolated Human Testicular Cells. Reproduction 2001, 121, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Alvarez, L.; Blank, N.; Balbach, M.; Turunen, H.; Laajala, T.D.; Toivanen, J.; Krutskikh, A.; Wahlberg, N.; Huhtaniemi, I.; et al. Targeted Inactivation of the Mouse Epididymal Beta-Defensin 41 Alters Sperm Flagellar Beat Pattern and Zona Pellucida Binding. Mol. Cell Endocrinol. 2016, 427, 143–154. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free Radicals: Their Beneficial and Detrimental Effects on Sperm Function. Indian. J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Aitken, R.J.; Baker, M.A. Oxidative Stress, Sperm Survival and Fertility Control. Mol. Cell Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative Stress and Male Infertility: Current Knowledge of Pathophysiology and Role of Antioxidant Therapy in Disease Management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gambera, L.; Stendardi, A.; Ghelardi, C.; Fineschi, B.; Aini, R. Effects of Antioxidant Treatment on Seminal Parameters in Patients Undergoing in Vitro Fertilization. Arch. Ital. Urol. E Androl. 2019, 91, 187. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic Review of Antioxidant Types and Doses in Male Infertility: Benefits on Semen Parameters, Advanced Sperm Function, Assisted Reproduction and Live-Birth Rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Agradi, S.; Curone, G.; Vigo, D.; Pastorelli, G.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Serra, V.; et al. Effect of Bovine Colostrum Dietary Supplementation on Rabbit Meat Quality. Foods 2022, 11, 3433. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gu, J.; Ma, W.; Zhang, Q.; Song, M.; Ha, L.; Xu, X.; Jiao, H.; Huo, Z. Lycium Barbarum Polysaccharide Protects against Ethanol-Induced Spermiotoxicity and Testicular Degeneration in Immp2l+/− Mice. Andrologia 2020, 52, e13554. [Google Scholar] [CrossRef]
- Jeong, H.C.; Jeon, S.H.; Guan Qun, Z.; Bashraheel, F.; Choi, S.W.; Kim, S.J.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Lycium chinense Mill Improves Hypogonadism via Anti-Oxidative Stress and Anti-Apoptotic Effect in Old Aged Rat Model. Aging Male 2020, 23, 287–296. [Google Scholar] [CrossRef]
- Varoni, M.V.; Gadau, S.D.; Pasciu, V.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Investigation of the Effects of Lycium barbarum Polysaccharides against Cadmium Induced Damage in Testis. Exp. Mol. Pathol. 2017, 103, 26–32. [Google Scholar] [CrossRef]
- Huang, X.; Yang, M.; Wu, X.; Yan, J. Study on Protective Action of Lycium barbarum Polysaccharides on DNA Imparments of Testicle Cells in Mice. Wei Sheng Yan Jiu 2003, 32, 599–601. [Google Scholar]
- Yang, Z.J.; Wang, Y.X.; Zhao, S.; Hu, N.; Chen, D.M.; Ma, H.M. SIRT 3 Was Involved in Lycium barbarum Seed Oil Protection Testis from Oxidative Stress: In Vitro and in Vivo Analyses. Pharm. Biol. 2021, 59, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Sheng, X.; Gambino, P.E.; Costello, B.; Bojanowski, K. Protective Effect of Fructus lycii Polysaccharides against Time and Hyperthermia-Induced Damage in Cultured Seminiferous epithelium. J. Ethnopharmacol. 2002, 82, 169–175. [Google Scholar] [CrossRef]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Protective Effects of Lycium barbarum Polysaccharide on Male Sexual Dysfunction and Fertility Impairments by Activating Hypothalamic Pituitary Gonadal Axis in Streptozotocin-Induced Type-1 Diabetic Male Mice. Endocr. J. 2017, 64, 907–922. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, X.; Yan, J.; Yang, M.; Liu, J.; Jiang, Y.; Li, J.; Zhou, Y. Antagonistic Effects of Lycium barbarum Polysaccharides on the Impaired Reproductive System of Male Rats Induced by Local Subchronic Exposure to 60Co-γ Irradiation. Phytother. Res. 2011, 25, 694–701. [Google Scholar] [CrossRef]
- Yang, F.L.; Wei, Y.X.; Liao, B.Y.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, J.L. Effects of Lycium barbarum Polysaccharide on Endoplasmic Reticulum Stress and Oxidative Stress in Obese Mice. Front. Pharmacol. 2020, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-F.; You, Z.-Q.; Gao, H.; Zhou, G.-L.; Chen, Y.; Yu, J.; Xuan, Y.-X. Protective Effect of Lycium barbarum Polysaccharides against Doxorubicin-Induced Testicular Toxicity in Rats. Phytother. Res. 2012, 26, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Zheng, G.; Chen, Y.; Huang, M.; Zhang, L.; Lin, X. Protective Effect of Lycium barbarum Polysaccharides against Cadmium-Induced Testicular Toxicity in Male Mice. Food Funct. 2017, 8, 2322–2330. [Google Scholar] [CrossRef]
- Zerani, M.; Boiti, C.; Dall’Aglio, C.; Pascucci, L.; Maranesi, M.; Brecchia, G.; Mariottini, C.; Guelfi, G.; Zampini, D.; Gobbetti, A. Leptin Receptor Expression and in Vitro Leptin Actions on Prostaglandin Release and Nitric Oxide Synthase Activity in the Rabbit Oviduct. J. Endocrinol. 2005, 185, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Boiti, C.; Guelfi, G.; Zerani, M.; Zampini, D.; Brecchia, G.; Gobbetti, A. Expression Patterns of Cytokines, P53 and Nitric Oxide Synthase Isoenzymes in Corpora lutea of Pseudopregnant Rabbits during Spontaneous luteolysis. Reproduction 2004, 127, 229–238. [Google Scholar] [CrossRef]
- Cremonesi, P.; Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Riva, F.; Marongiu, M.L.; Castiglioni, B.; Barbato, O.; Munga, A.; et al. Dietary Supplementation with Goji Berries (Lycium barbarum) Modulates the Microbiota of Digestive Tract and Caecal Metabolites in Rabbits. Animals 2022, 12, 121. [Google Scholar] [CrossRef]
- Boiti, C.; Guelfi, G.; Zampini, D.; Brecchia, G.; Gobbetti, A.; Zerani, M. Regulation of Nitric Oxide Synthase Isoforms and Role of Nitric Oxide during Prostaglandin F2alpha-Induced Luteolysis in Rabbits. Reproduction 2003, 125, 807–816. [Google Scholar] [CrossRef]
- Zerani, M.; Parillo, F.; Brecchia, G.; Guelfi, G.; Dall’Aglio, C.; Lilli, L.; Maranesi, M.; Gobbetti, A.; Boiti, C. Expression of Type I GNRH Receptor and in Vivo and in Vitro GNRH-I Effects in Corpora lutea of Pseudopregnant Rabbits. J. Endocrinol. 2010, 207, 289–300. [Google Scholar] [CrossRef]
- Andoni, E.; Curone, G.; Agradi, S.; Barbato, O.; Menchetti, L.; Vigo, D.; Zelli, R.; Cotozzolo, E.; Ceccarini, M.R.; Faustini, M.; et al. Effect of Goji Berry (Lycium barbarum) Supplementation on Reproductive Performance of Rabbit Does. Animals 2021, 11, 1672. [Google Scholar] [CrossRef]
- Brecchia, G.; Sulce, M.; Curone, G.; Barbato, O.; Canali, C.; Troisi, A.; Munga, A.; Polisca, A.; Agradi, S.; Ceccarini, M.R.; et al. Goji Berry (Lycium barbarum) Supplementation during Pregnancy Influences Insulin Sensitivity in Rabbit Does but Not in Their Offspring. Animals 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Curone, G.; Andoni, E.; Barbato, O.; Troisi, A.; Fioretti, B.; Polisca, A.; Codini, M.; Canali, C.; Vigo, D.; et al. Impact of Goji Berries (Lycium barbarum) Supplementation on the Energy Homeostasis of Rabbit Does: Uni- and Multivariate Approach. Animals 2020, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Lee, S.H.; Lee, J.M.; Jerng, U.M. Semen Quality Improvement in a Man with Idiopathic Infertility Treated with Traditional Korean Medicine: A Case Report. Explor. J. Sci. Health 2015, 11, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, Z.; Huang, X.; Yan, J.; Zhang, S.; Cai, Y.Z. Lycium barbarum Polysaccharides: Protective Effects against Heat-Induced Damage of Rat Testes and H2O2-Induced DNA Damage in Mouse Testicular Cells and Beneficial Effect on Sexual Behavior and Reproductive Function of Hemicastrated Rats. Life Sci. 2006, 79, 613–621. [Google Scholar] [CrossRef]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Beneficial Effects of Lycium barbarum Polysaccharide on Spermatogenesis by Improving Antioxidant Activity and Inhibiting Apoptosis in Streptozotocin-Induced Diabetic Male Mice. Food Funct. 2017, 8, 1215–1226. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, Q.; Zhang, S.H.; Mu, C.L.; Yao, P.; Jiao, H.Y.; Xu, X.; Huo, Z.H. Lycium Barbarum Polysaccharide Reduces Testicular Spermatogenic Injury in Immp2l−/− mice through GPX4 and AIF Pathways. Zhonghua Nan Ke Xue 2021, 27, 387–393. [Google Scholar]
- Yang, Q.; Xing, Y.; Qiao, C.; Liu, W.; Jiang, H.; Fu, Q.; Zhou, Y.; Yang, B.; Zhang, Z.; Chen, R. Semen Quality Improvement in Boars Fed with Supplemental Wolfberry (Lycium barbarum). Anim. Sci. J. 2019, 90, 1517–1522. [Google Scholar] [CrossRef]
- Ren, F.; Fang, Q.; Feng, T.; Li, Y.; Wang, Y.; Zhu, H.; Hu, J. Lycium Barbarum and Laminaria Japonica Polysaccharides Improve Cashmere Goat Sperm Quality and Fertility Rate after Cryopreservation. Theriogenology 2019, 129, 29–36. [Google Scholar] [CrossRef]
- Castellini, C.; Ruggeri, S.; Mattioli, S.; Bernardini, G.; Macchioni, L.; Moretti, E.; Collodel, G. Long-Term Effects of Silver Nanoparticles on Reproductive Activity of Rabbit Buck. Syst. Biol. Reprod. Med. 2014, 60, 143–150. [Google Scholar] [CrossRef]
- Menchetti, L.; Brecchia, G.; Branciari, R.; Barbato, O.; Fioretti, B.; Codini, M.; Bellezza, E.; Trabalza-Marinucci, M.; Miraglia, D. The Effect of Goji Berries (Lycium barbarum) Dietary Supplementation on Rabbit Meat Quality. Meat Sci. 2020, 161, 108018. [Google Scholar] [CrossRef]
- Maertens, L.; Moermans, R.; De Groote, G. Prediction of the Apparent Digestible Energy Content of Commercial Pelleted Feeds for Rabbits. J. Appl. Rabbit. Res. 1988, 11, 60–67. [Google Scholar]
- Fallas-López, M.; Rodríguez-De Lara, R.; Bárcena-Gama, R.; Sánchez-Torres Esqueda, M.T.; Hernández-Sánchez, D.; Martínez-Hernández, P.A.; Aguilar-Romero, O. Rabbit Sexual Behavior, Semen and Sperm Characteristics When Supplemented with Sprouted Wheat. Anim. Reprod. Sci. 2011, 129, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Garson, G.D. Measures of Association (Statistical Associates “Blue Book” Series 8) (English Edition), 1st ed.; Statistical Associates Publishers: Washington, DC, USA, 2012. [Google Scholar]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using SPSS, 3rd ed.SAGE Publications: London, UK, 2009; Volume 81, ISBN 9781847879066. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE Publications: London, UK, 2013; Volume 58. [Google Scholar]
- Carnés, J.; De Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; López-Matas, M.A.; Pagán, J.A.; Navarro, L.A.; García-Abujeta, J.L.; Vicario, S.; Peña, M. Recently Introduced Foods as New Allergenic Sources: Sensitisation to Goji Berries (Lycium barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef]
- Lei, X.; Huo, P.; Wang, Y.; Xie, Y.; Shi, Q.; Tu, H.; Yao, J.; Mo, Z.; Zhang, S. Lycium Barbarum Polysaccharides Improve Testicular Spermatogenic Function in Streptozotocin-Induced Diabetic Rats. Front. Endocrinol. 2020, 11, 164. [Google Scholar] [CrossRef]
- Dursun, R.; Zengin, Y.; Gündüz, E.; İçer, M.; Durgun, H.M.; Dağgulli, M.; Kaplan, I.; Alabalık, U.; Güloğlu, C. The Protective Effect of Goji Berry Extract in Ischemic Reperfusion in Testis Torsion. Int. J. Clin. Exp. Med. 2015, 8, 2727–2733. [Google Scholar] [PubMed]
- Zhang, C.; Wang, A.; Sun, X.; Li, X.; Zhao, X.; Li, S.; Ma, A. Protective Effects of Lycium barbarum Polysaccharides on Testis Spermatogenic Injury Induced by Bisphenol a in Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 690808. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Cui, X.; Yan, J.; Zhao, Q.; Xiang, C. The Effect of Lycium Barbarum Polysaccharides on the Male Rats’ Reproductive System and Spermatogenic Cell Apoptosis Exposed to Low-Dose Ionizing Irradiation. J. Ethnopharmacol. 2014, 154, 249–258. [Google Scholar] [CrossRef]
- Rasooli, A.; Taha Jalali, M.; Nouri, M.; Mohammadian, B.; Barati, F. Effects of Chronic Heat Stress on Testicular Structures, Serum Testosterone and Cortisol Concentrations in Developing Lambs. Anim. Reprod. Sci. 2010, 117, 55–59. [Google Scholar] [CrossRef]
- Qian, L.; Yu, S. Protective Effect of Polysaccharides from Lycium Barbarum on Spermatogenesis of Mice with Impaired Reproduction System Induced by Cyclophosphamide. Am. J. Reprod. Immunol. 2016, 76, 383–385. [Google Scholar] [CrossRef]
- Dalton, J.C. Semen Quality Factors Associated with Fertility. In Proceedings of the Applied Reproductive Strategies in Beef Cattle—Northwest, Boise, ID, USA, 30 September–1 October 2011. [Google Scholar]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review Andmeta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus Faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed]
- Taepongsorat, L.; Tangpraprutgul, P.; Kitana, N.; Malaivijitnond, S. Stimulating Effects of Quercetin on Sperm Quality and Reproductive Organs in Adult Male Rats. Asian J. Androl. 2008, 10, 249–258. [Google Scholar] [CrossRef]
- Diao, R.; Gan, H.; Tian, F.; Cai, X.; Zhen, W.; Song, X.; Duan, Y. In Vitro Antioxidation Effect of Quercetin on Sperm Function from the Infertile Patients with Leukocytospermia. Am. J. Reprod. Immunol. 2019, 82, e13155. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Goyal, A.; Delves, G.H.; Chopra, M.; Lwaleed, B.A.; Cooper, A.J. Can Lycopene Be Delivered into Semen via Prostasomes? In Vitro Incorporation and Retention Studies. Int. J. Androl. 2006, 29, 528–533. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant Efficiency of Lycopene on Oxidative Stress—Induced Damage in Bovine Spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 50. [Google Scholar] [CrossRef]





| Ingredients/Analytical Data | Diet | |
|---|---|---|
| Control | Goji | |
| Ingredients 1 | ||
| Wheat bran | 30.0 | 29.5 |
| Dehydrated alfalfa meal | 42.0 | 41.5 |
| Barley | 9.6 | 9.6 |
| Sunflower meal | 4.6 | 4.6 |
| Rice bran | 4.0 | 4.0 |
| Soybean meal | 4.0 | 4.0 |
| Calcium carbonate | 2.0 | 2.0 |
| Cane molasses | 2.0 | 2.0 |
| Dicalcium phosphate | 0.7 | 0.7 |
| Vitamin–mineral premix 2 | 0.4 | 0.4 |
| Soybean oil | 0.4 | 0.4 |
| Salt | 0.3 | 0.3 |
| Goji berries | - | 1 |
| Analytical data 1 | ||
| Crude protein | 15.74 | 15.64 |
| Ether extract | 2.25 | 2.23 |
| Ash | 9.28 | 9.36 |
| Starch | 16.86 | 17.07 |
| NDF | 38.05 | 38.55 |
| ADF | 19.54 | 19.60 |
| ADL | 4.01 | 4.31 |
| Digestible Energy 3 | 2464 | 2463 |
| Parameter | Time | Group | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Goji | Group | Time | Group × Time | ||
| Volume (mL) | T1 | 0.95 ± 0.19 | 0.97 ± 0.07 | 0.791 | 0.733 | 0.413 |
| T2 | 0.98 ± 0.12 | 0.80 ± 0.12 | ||||
| T3 | 0.83 ± 0.10 | 1.01 ± 0.09 | ||||
| T4 | 0.98 ± 0.08 | 1.03 ± 0.17 | ||||
| T5 | 0.96 ± 0.16 | 1.03 ± 0.06 | ||||
| Marginal means ± SE | 0.94 ± 0.06 | 0.97 ± 0.05 | ||||
| Concentration (spermatozoa/mL; 106) | T1 | 189 ± 36 | 263 ± 36 | 0.040 | 0.140 | 0.339 |
| T2 | 176 ± 33 | 309 ± 49 | ||||
| T3 | 253 ± 45 | 302 ± 23 | ||||
| T4 | 249 ± 47 | 310 ± 33 | ||||
| T5 | 268 ± 50 | 354 ± 45 | ||||
| Marginal means ± SE | 227 a ± 19 | 308 b ± 17 | ||||
| Motility (%) | T1 | 81 ± 6 | 95 ± 1 | 0.001 | 0.081 | 0.096 |
| T2 | 87 ± 4 | 94 ± 1 | ||||
| T3 | 88 ± 3 | 94 ± 1 | ||||
| T4 | 87 ± 3 | 92 ± 3 | ||||
| T5 | 93 ± 1 | 94 ± 2 | ||||
| Marginal means ± SE | 87 a ± 2 | 94 b ± 1 | ||||
| Live spermatozoa (%) | T1 | 91.1 ± 4.0 | 97.0 ± 0.6 | 0.010 | 0.283 | 0.003 |
| T2 | 95.0 ± 1.5 | 92.4 ± 2.8 | ||||
| T3 | 93.2 ± 1.8 | 95.1 ± 1.1 | ||||
| T4 | 95.2 ± 1.0 | 97.6 ± 0.5 | ||||
| T5 | 93.1 ± 1.3 | 94.1 ± 2.0 | ||||
| Marginal means ± SE | 93.5 a ± 1.0 | 95.2 b ± 0.8 | ||||
| Abnormal spermatozoa (%) | T1 | 7.6 ± 1.2 | 5.3 ± 1.1 | <0.001 | <0.001 | 0.277 |
| T2 | 7.9 ± 0.9 | 6.6 ± 1.1 | ||||
| T3 | 8.6 ± 2.2 | 4.6 ± 1.5 | ||||
| T4 | 19.2 ± 2.1 | 11.2 ± 1.5 | ||||
| T5 | 13.2 ± 0.8 | 9.9 ± 1.3 | ||||
| Marginal means ± SE | 11.3 b ± 0.9 | 7.5 a ± 0.7 | ||||
| Reaction time (s) * | T1 | 11.11 ± 2.89 | 6.52 ± 1.74 | 0.066 | <0.001 | 0.340 |
| T2 | 4.50 ± 1.33 | 4.27 ± 2.81 | ||||
| T3 | 5.59 ± 1.88 | 1.88 ± 0.44 | ||||
| T4 | 2.99 ± 1.75 | 1.89 ± 0.66 | ||||
| T5 | 3.23 ± 0.99 | 0.99 ± 0.23 | ||||
| Marginal means ± SE | 5.48 ± 0.92 | 3.02 ± 0.77 | ||||
| Parameter | Time | Group | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Goji | Group | Time | Group × Time | ||
| TAC | T1 | 4.32 ± 0.74 | 5.78 ± 1.00 | 0.808 | <0.001 | <0.001 |
| T2 | 4.79 ± 0.80 | 3.71 ± 0.59 | ||||
| T3 | 4.51 ± 0.45 | 6.38 ± 0.45 | ||||
| T4 | 5.88 ± 0.21 | 4.52 ± 0.32 | ||||
| T5 | 7.42 ± 0.67 | 7.17 ± 0.46 | ||||
| Marginal means ± SE | 5.38 ± 0.31 | 5.51 ± 0.33 | ||||
| CAT | T1 | 161.58 ± 36.27 | 294.60 ± 82.54 | 0.296 | 0.004 | <0.001 |
| T2 | 109.96 ± 19.63 | 169.99 ± 37.63 | ||||
| T3 | 151.16 ± 27.85 | 190.70 ± 23.51 | ||||
| T4 | 150.00 ± 17.72 | 130.47 ± 33.58 | ||||
| T5 | 190.94 ± 39.92 | 171.74 ± 34.39 | ||||
| Marginal means ± SE | 152.73 ± 13.22 | 192.59 ± 22.40 | ||||
| SOD | T1 | 150.42 ± 19.61 | 173.36 ± 15.26 | 0.115 | 0.261 | 0.008 |
| T2 | 169.52 ± 19.06 | 165.70 ± 28.08 | ||||
| T3 | 194.43 ± 27.69 | 161.23 ± 20.48 | ||||
| T4 | 195.05 ± 16.33 | 161.41 ± 11.13 | ||||
| T5 | 212.45 ± 31.38 | 158.22 ± 15.65 | ||||
| Marginal means ± SE | 184.38 ± 10.55 | 163.98 ± 8.10 | ||||
| GPx (U/mL) | T1 | 47.54 ± 24.85 | 182.42 ± 54.69 | 0.084 | 0.565 | 0.094 |
| T2 | 44.15 ± 12.07 | 86.23 ± 21.33 | ||||
| T3 | 137.53 ± 44.84 | 210.12 ± 98.47 | ||||
| T4 | 51.79 ± 19.01 | 135.62 ± 35.89 | ||||
| T5 | 75.56 ± 16.67 | 85.00 ± 38.92 | ||||
| Marginal means ± SE | 71.31 ± 12.32 | 141.26 ± 25.78 | ||||
| IL-1β (pg/mL) | T1 | 1698.47 ± 200.79 | 1901.66 ± 199.64 | 0.630 | 0.561 | 0.205 |
| T2 | 1594.85 ± 264.49 | 1834.93 ± 250.40 | ||||
| T3 | 1742.88 ± 274.36 | 1887.11 ± 229.48 | ||||
| T4 | 1998.12 ± 184.93 | 1740.87 ± 208.84 | ||||
| T5 | 1833.63 ± 183.01 | 1945.94 ± 150.24 | ||||
| Marginal means ± SE | 1775.30 ± 98.66 | 1862.10 ± 89.86 | ||||
| Concentration | Motility | Live Spermatozoa | Abnormal Spermatozoa | Reaction Time | TAC | CAT | SOD | GPx | IL-1β | |
|---|---|---|---|---|---|---|---|---|---|---|
| Volume | 0.182 | 0.131 | 0.233 * | −0.118 | 0.025 | 0.219 * | −0.074 | 0.031 | 0.070 | 0.091 |
| Concentration | 0.572 ** | 0.236 * | −0.195 | −0.256 * | −0.025 | 0.045 | −0.008 | 0.233 * | 0.213 | |
| Motility | 0.348 ** | −0.214 * | 0.027 | 0.015 | 0.065 | −0.057 | 0.171 | 0.086 | ||
| Live spermatozoa | −0.382 ** | 0.020 | −0.014 | 0.118 | 0.020 | 0.237 * | 0.288 ** | |||
| Abnormal spermatozoa | −0.214 | 0.077 | −0.108 | −0.056 | −0.279 * | −0.143 | ||||
| Reaction time | −0.082 | −0.011 | −0.171 | −0.205 | −0.178 | |||||
| TAC | 0.422 ** | 0.150 | 0.121 | 0.133 | ||||||
| CAT | 0.262 * | 0.365 ** | 0.131 | |||||||
| SOD | 0.346 ** | 0.162 | ||||||||
| GPx | 0.265 * |
| Tissue | Parameter | Volume | Concentration | Motility | Live Spermatozoa | Abnormal Spermatozoa | Reaction Time | TAC | CAT | SOD | GPx | IL-1β |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epididymis | Degeneration | 0.26 | 0.489 * | 0.324 | 0.568 * | −0.251 | −0.293 | 0.216 | 0.399 | −0.175 | 0.192 | 0.011 |
| Functional activity | −0.133 | 0.229 | 0.453 | 0.164 | −0.507 * | −0.13 | 0.12 | 0.284 | −0.248 | 0.395 | 0.113 | |
| Hyperplasia | 0.222 | 0.214 | 0.443 | 0.639 ** | −0.313 | −0.138 | 0.542 * | 0.535 * | −0.374 | 0.374 | 0.351 | |
| Testis | Degeneration | −0.606 ** | −0.046 | −0.334 | −0.164 | 0.299 | −0.12 | −0.253 | −0.017 | 0.148 | −0.24 | −0.413 |
| Functional activity | −0.037 | 0.242 | 0.595 * | 0.273 | −0.501 * | −0.277 | 0.088 | 0.327 | −0.493 * | 0.452 | −0.121 | |
| Hyperplasia | −0.277 | 0.108 | 0.150 | 0.276 | −0.389 | −0.055 | 0.284 | 0.482 | −0.161 | 0.223 | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brecchia, G.; Muça, G.; Munga, A.; Menchetti, L.; Galosi, L.; Rossi, G.; Barbato, O.; Pastorelli, G.; Agradi, S.; Serra, V.; et al. Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract. Antioxidants 2023, 12, 1959. https://doi.org/10.3390/antiox12111959
Brecchia G, Muça G, Munga A, Menchetti L, Galosi L, Rossi G, Barbato O, Pastorelli G, Agradi S, Serra V, et al. Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract. Antioxidants. 2023; 12(11):1959. https://doi.org/10.3390/antiox12111959
Chicago/Turabian StyleBrecchia, Gabriele, Gerald Muça, Albana Munga, Laura Menchetti, Livio Galosi, Giacomo Rossi, Olimpia Barbato, Grazia Pastorelli, Stella Agradi, Valentina Serra, and et al. 2023. "Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract" Antioxidants 12, no. 11: 1959. https://doi.org/10.3390/antiox12111959
APA StyleBrecchia, G., Muça, G., Munga, A., Menchetti, L., Galosi, L., Rossi, G., Barbato, O., Pastorelli, G., Agradi, S., Serra, V., Sulçe, M., Ozuni, E., Turmalaj, L., Castrica, M., Ceccarini, M. R., Riva, F., Fioretti, B., Quattrone, A., Marongiu, M. L., & Curone, G. (2023). Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract. Antioxidants, 12(11), 1959. https://doi.org/10.3390/antiox12111959