Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments
Abstract
:1. Introduction
2. Food Biotechnology and Conventional Food Processing for Human Well-Being
3. Polyphenols in the Food Industry and Human Health
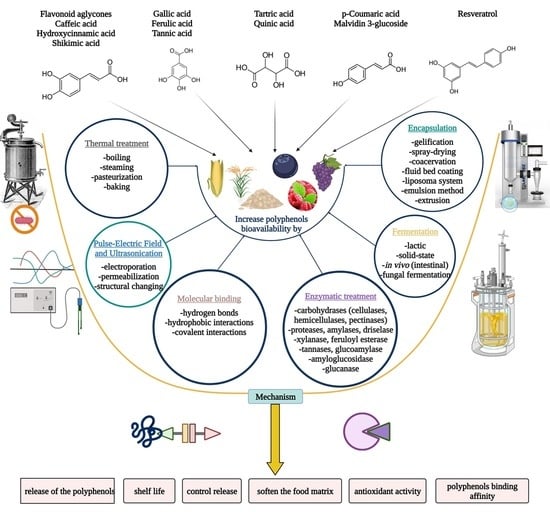
4. Techniques That Boost Polyphenols Availability
4.1. Non-Chemical Technologies
4.1.1. Thermal Treatment
4.1.2. Pulsed Electric Field (PEF) and Ultrasonic Techniques
4.2. Chemical Technologies
Molecular Binding
4.3. Biotechnological Technologies
4.3.1. Enzymatic Treatments
4.3.2. Fermentation
4.3.3. Encapsulation
5. Future Opportunities to Increase Accessibility of Polyphenols from Food
6. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fărcaș, A.; Drețcanu, G.; Pop, T.D.; Enaru, B.; Socaci, S.; Diaconeasa, Z. Cereal processing by-products as rich sources of phenolic compounds and their potential bioactivities. Nutrients 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile profile, fatty acids composition and total phenolics content of brewers’ spent grain by-product with potential use in the development of new functional foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Johansson, E.; Hussain, A.; Kuktaite, R.; Andersson, S.C.; Olsson, M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health 2014, 11, 3870–3893. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Rojas, P.; Stucken, K.; Delporte, C.; Valenzuela-Barra, G.; Jagus, R.J.; Agüero, M.V.; Pasten, A. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J. Appl. Res. Med. Aromat. Plants 2018, 11, 37–46. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2017, 244, 735–745. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ranga, F.; Rugină, D.; Leopold, L.; Oana, P.; Vodnar, D.; Cuibus, L.; Socaciu, C. Phenolic content and their antioxidant activity in various berries cultivated in Romania. Bull. UASVM Food Sci. Technol. 2015, 72, 1. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Xue, R.; Hu, R. The neuroprotective effect and action mechanism of polyphenols in diabetes mellitus-related cognitive dysfunction. Eur. J. Nutr. 2020, 59, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Bishayee, A.; Yousefi, B. Polyphenols: Major regulators of key components of DNA damage response in cancer. DNA Repair 2019, 82, 102679. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Estévez, M.; Barba, F.J.; Thirumdas, R.; Franco, D.; Munekata, P.E.S. Polyphenols: Bioaccessibility and bioavailability of bioactive components. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–332. [Google Scholar]
- Knorr, D.; Khoo, C.S.H.; Augustin, M.A. Food for an Urban Planet: Challenges and Research Opportunities. Front. Nutr. 2017, 4, 73. [Google Scholar] [CrossRef]
- Jurabaevich, S.N.; Bulturbayevich, M.B. Directions for food security in the context of globalization. Inn. Technol. Methodical Res. J. 2021, 1, 9–16. [Google Scholar]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Kuddus, M. Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- El Sheikha, A.F.; Levin, R.E.; Xu, J. Molecular Techniques in Food Biology: Safety, Biotechnology, Authenticity and Traceability; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kallscheuer, N.; Classen, T.; Drepper, T.; Marienhagen, J. Production of plant metabolites with applications in the food industry using engineered microorganisms. Curr. Opin. Biotechnol. 2019, 56, 7–17. [Google Scholar] [CrossRef]
- Martinelli, L.; Karbarz, M.; Siipi, H. Science, safety, and trust: The case of transgenic food. Croat. Med. J. 2013, 54, 91. [Google Scholar] [CrossRef]
- Battacchi, D.; Verkerk, R.; Pellegrini, N.; Fogliano, V.; Steenbekkers, B. The state of the art of food ingredients’ naturalness evaluation: A review of proposed approaches and their relation with consumer trends. Trends Food Sci. Technol. 2020, 106, 434–444. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Barbaccia, P.; Francesca, N.; Gerlando, R.D.; Busetta, G.; Moschetti, G.; Gaglio, R.; Settanni, L. Biodiversity and dairy traits of indigenous milk lactic acid bacteria grown in presence of the main grape polyphenols. FEMS Microbiol. Lett. 2020, 367, fnaa066. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Fărcaş, A.; Socaci, S.; Vodnar, D.C. Innovative sources. In Nutraceuticals and Natural Product Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–265. [Google Scholar]
- Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; Dulf, F.V.; Vodnar, D.C.; Tofană, M.; Salanță, L.C. Exploitation of brewing industry wastes to produce functional ingredients. In Brewing Technology; IntechOpen: London, UK, 2017; pp. 137–156. [Google Scholar]
- Vagiri, M.; Ekholm, A.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Major phenolic compounds in black currant (Ribes nigrum L.) buds: Variation due to genotype, ontogenetic stage and location. LWT-Food Sci. Technol. 2015, 63, 1274–1280. [Google Scholar] [CrossRef]
- Liu, D.; Martinez-Sanz, M.; Lopez-Sanchez, P.; Gilbert, E.P.; Gidley, M.J. Adsorption behaviour of polyphenols on cellulose is affected by processing history. Food Hydrocoll. 2017, 63, 496–507. [Google Scholar] [CrossRef]
- Boz, H. Ferulic Acid in Cereals—A Review. Czech J. Food Sci. 2015, 33. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Socaci, S.A.; Rugină, D.O.; Diaconeasa, Z.M.; Pop, O.L.; Fărcaș, A.C.; Păucean, A.; Tofană, M.; Pintea, A. Antioxidant compounds recovered from food wastes. In Functional Food—Improve Health through Adequate Food; IntechOpen: London, UK, 2017; pp. 3–22. [Google Scholar]
- Veiga-Santos, P.; Silva, L.T.; de Souza, C.O.; da Silva, J.R.; Albuquerque, E.C.; Druzian, J.I. Coffee-cocoa additives for bio-based antioxidant packaging. Food Packag. Shelf Life 2018, 18, 37–41. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 223. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Pepé Sciarria, T.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankowski, R.; You, L.; Buford, T.; Leeuwenburgh, C.; Manini, T.; Schneider, S.; Qiu, P.; Anton, S. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Chen, Z.; Luo, X.; Li, Y.; Wang, R.; Li, J.; Li, Y.; Wang, T.; Wu, J. Effects of milk proteins on the bioaccessibility and antioxidant activity of oat phenolics during in vitro digestion. J. Food Sci. 2019, 84, 895–903. [Google Scholar] [CrossRef]
- Paramanantham, A.; Kim, M.J.; Jung, E.J.; Nagappan, A.; Yun, J.W.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Lee, W.S. Pretreatment of anthocyanin from the fruit of vitis coignetiae pulliat acts as a potent inhibitor of TNF-α effect by inhibiting NF-κB-regulated genes in human breast cancer cells. Molecules 2020, 25, 2396. [Google Scholar] [CrossRef]
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural polyphenols targeting senescence: A novel prevention and therapy strategy for cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The protective effect of polyphenols for colorectal cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Norouzi, S.; Majeed, M.; Pirro, M.; Generali, D.; Sahebkar, A. Curcumin as an adjunct therapy and microRNA modulator in breast cancer. Curr. Pharm. Des. 2018, 24, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Meeran, S.M.; Katiyar, S.K. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014, 355, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Penta, D.; Mondal, P.; Meeran, S.M. Epigenetics of breast cancer: Clinical status of epi-drugs and phytochemicals. In Breast Cancer Metastasis and Drug Resistance: Challenges and Progress; Springer: Cham, Switzerland, 2019; pp. 293–310. [Google Scholar]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Sorrentino, C.; Di Gisi, M.; Gentile, G.; Licitra, F.; D’Angiolo, R.; Giovannelli, P.; Migliaccio, A.; Castoria, G.; Di Donato, M. Agri-Food By-Products in Cancer: New Targets and Strategies. Cancers 2022, 14, 5517. [Google Scholar] [CrossRef]
- Kayath, C.A.; Ibala Zamba, A.; Mokémiabeka, S.N.; Opa-Iloy, M.; Elenga Wilson, P.S.; Kaya-Ongoto, M.D.; Mouellet Maboulou, R.J.; Nguimbi, E. Synergic Involvements of Microorganisms in the Biomedical Increase of Polyphenols and Flavonoids during the Fermentation of Ginger Juice. Int. J. Microbiol. 2020, 2020, 8417693. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [Green Version]
- Polia, F.; Pastor-Belda, M.; Martinez-Blazquez, A.; Horcajada, M.N.; Tomas-Barberan, F.A.; Garcia-Villalba, R. Technological and Biotechnological Processes To Enhance the Bioavailability of Dietary (Poly)phenols in Humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Martins, I.M.; Roberto, B.S.; Blumberg, J.B.; Chen, C.-Y.O.; Macedo, G.A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Int. 2016, 89, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jayaprakasha, G.K.; Patil, B.S. Encapsulation of Polyphenols: An Effective Way To Enhance Their Bioavailability for Gut Health. In Advances in Plant Phenolics: From Chemistry to Human Healt; American Chemical Society: Washington, DC, USA, 2018; pp. 239–259. [Google Scholar]
- Barba, F.J.; Saraiva, J.M.A.; Cravotto, G.; Lorenzo, J.M. Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- David-Birman, T.; Raften, G.; Lesmes, U. Effects of thermal treatments on the colloidal properties, antioxidant capacity and in-vitro proteolytic degradation of cricket flour. Food Hydrocoll. 2018, 79, 48–54. [Google Scholar] [CrossRef]
- Kubo, M.T.; Baicu, A.; Erdogdu, F.; Poças, M.F.; Silva, C.L.; Simpson, R.; Vitali, A.A.; Augusto, P.E. Thermal processing of food: Challenges, innovations and opportunities. A position paper. Food Rev. Int. 2021, 1–26. [Google Scholar] [CrossRef]
- Stübler, A.-S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of pilot-scale processing (thermal, PEF, HPP) on the stability and bioaccessibility of polyphenols and proteins in mixed protein-and polyphenol-rich juice systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Tembo, D.T.; Holmes, M.J.; Marshall, L.J. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J. Food Compost. Anal. 2017, 58, 40–51. [Google Scholar] [CrossRef] [Green Version]
- de Lima, A.C.S.; da Rocha Viana, J.D.; de Sousa Sabino, L.B.; da Silva, L.M.R.; da Silva, N.K.V.; de Sousa, P.H.M. Processing of three different cooking methods of cassava: Effects on in vitro bioaccessibility of phenolic compounds and antioxidant activity. LWT-Food Sci. Technol. 2017, 76, 253–258. [Google Scholar] [CrossRef]
- Mennah-Govela, Y.A.; Bornhorst, G.M. Fresh-squeezed orange juice properties before and during in vitro digestion as influenced by orange variety and processing method. J. Food Sci. 2017, 82, 2438–2447. [Google Scholar] [CrossRef]
- Tomas, M.; Rocchetti, G.; Ghisoni, S.; Giuberti, G.; Capanoglu, E.; Lucini, L. Effect of different soluble dietary fibres on the phenolic profile of blackberry puree subjected to in vitro gastrointestinal digestion and large intestine fermentation. Food Res. Int. 2020, 130, 108954. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.D.; Beekwilder, J.; Capanoglu, E. Processing black mulberry into jam: Effects on antioxidant potential and in vitro bioaccessibility. J. Sci. Food Agric. 2017, 97, 3106–3113. [Google Scholar] [CrossRef]
- Cheng, J.-R.; Xiang, R.; Liu, X.-M.; Zhu, M.-J. The effects of thermal processing and β-cyclodextrin on extractable polyphenols in mulberry juice-enriched dried minced pork slices. LWT-Food Sci. Technol. 2019, 116, 108503. [Google Scholar] [CrossRef]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ribeiro, L.; Pinheiro, A.C.B.; Santa Brígida, A.I.; Genisheva, Z.A.; de Oliveira Soares, A.A.M.; Teixeira, J.A.C.; de Matta, V.M.; Freitas, S.P. In vitro gastrointestinal evaluation of a juçara-based smoothie: Effect of processing on phenolic compounds bioaccessibility. J. Food Sci. Food Sci. Technol. 2019, 56, 5017–5026. [Google Scholar] [CrossRef] [Green Version]
- Calinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonteles, T.V.; Leite, A.K.F.; Silva, A.R.A.; Carneiro, A.P.G.; de Castro Miguel, E.; Cavada, B.S.; Fernandes, F.A.N.; Rodrigues, S. Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016, 31, 237–249. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Toepfl, S.; Siemer, C.; Saldaña-Navarro, G.; Heinz, V. Overview of pulsed electric fields processing for food. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 93–114. [Google Scholar]
- Schilling, S.; Schmid, S.; Jäger, H.; Ludwig, M.; Dietrich, H.; Toepfl, S.; Knorr, D.; Neidhart, S.; Schieber, A.; Carle, R. Comparative study of pulsed electric field and thermal processing of apple juice with particular consideration of juice quality and enzyme deactivation. J. Agricult. Food Chem. 2008, 56, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Grimi, N.; Mamouni, F.; Lebovka, N.; Vorobiev, E.; Vaxelaire, J. Impact of apple processing modes on extracted juice quality: Pressing assisted by pulsed electric fields. J. Food Eng. 2011, 103, 52–61. [Google Scholar] [CrossRef]
- Turk, M.F.; Vorobiev, E.; Baron, A. Improving apple juice expression and quality by pulsed electric field on an industrial scale. LWT-Food Sci. Technol. 2012, 49, 245–250. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Schilling, S.; Alber, T.; Toepfl, S.; Neidhart, S.; Knorr, D.; Schieber, A.; Carle, R. Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 127–134. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Nemes, S.A.; Calinoiu, L.F.; Dulf, F.V.; Farcas, A.C.; Vodnar, D.C. Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxidants 2022, 11, 2159. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and β-glucan contents: Compositional and kinetic studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Berndtsson, E.; Andersson, R.; Johansson, E.; Olsson, M.E. Side streams of broccoli leaves: A climate smart and healthy food ingredient. Int. J. Environ. Res. Public Health 2020, 17, 2406. [Google Scholar] [CrossRef] [Green Version]
- Poklar Ulrih, N. Analytical techniques for the study of polyphenol–protein interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 2144–2161. [Google Scholar] [CrossRef]
- Rasheed, R.A.; Kamsin, A.; Abdullah, N.A. Challenges in the online component of blended learning: A systematic review. Comput. Educ. 2020, 144, 103701. [Google Scholar] [CrossRef]
- Fan, D.-M.; Fan, K.; Yu, C.-P.; Lu, Y.-T.; Wang, X.-C. Tea polyphenols dominate the short-term tea (Camellia sinensis) leaf litter decomposition. J. Zhejiang Univ. Sci. B 2017, 18, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, Y.; Liu, L.; Gong, Y.; Xie, Y.; Cao, Y. Chemical structures of polyphenols that critically influence the toxicity of ZnO nanoparticles. J. Agric. Food Chem. 2018, 66, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Morariu, I.D.; Avasilcăi, L.; Vieriu, M.; Cioancă, O.; Hăncianu, M. Immunochemical assay of chloramphenicol in honey. Farmacia 2019, 67, 235–239. [Google Scholar] [CrossRef]
- Gu, C.; Suleria, H.A.; Dunshea, F.R.; Howell, K. Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion. Antioxidants 2020, 9, 762. [Google Scholar] [CrossRef]
- Fu, S.; Augustin, M.A.; Sanguansri, L.; Shen, Z.; Ng, K.; Ajlouni, S. Enhanced bioaccessibility of curcuminoids in buttermilk yogurt in comparison to curcuminoids in aqueous dispersions. J. Food Sci. 2016, 81, H769–H776. [Google Scholar] [CrossRef]
- Ferraro, V.; Madureira, A.R.; Sarmento, B.; Gomes, A.; Pintado, M.E. Study of the interactions between rosmarinic acid and bovine milk whey protein α-Lactalbumin, β-Lactoglobulin and Lactoferrin. Food Res. Int. 2015, 77, 450–459. [Google Scholar] [CrossRef]
- von Staszewski, M.; Jara, F.L.; Ruiz, A.L.; Jagus, R.J.; Carvalho, J.E.; Pilosof, A.M. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Al-Hanish, A.; Stanic-Vucinic, D.; Mihailovic, J.; Prodic, I.; Minic, S.; Stojadinovic, M.; Radibratovic, M.; Milcic, M.; Velickovic, T.C. Noncovalent interactions of bovine α-lactalbumin with green tea polyphenol, epigalocatechin-3-gallate. Food Hydrocoll. 2016, 61, 241–250. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Alsenaidy, M.A.; Rehman, M.T.; AlAjmi, M.F.; Alsenaidy, A.M.; Husain, F.M.; Khan, R.H. Molecular insight into binding behavior of polyphenol (rutin) with beta lactoglobulin: Spectroscopic, molecular docking and MD simulation studies. J. Mol. Liquids 2018, 269, 511–520. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, B.; Wang, Y.; Teng, Y. Effects of colloidal complexes formation between resveratrol and deamidated gliadin on the bioaccessibility and lipid oxidative stability. Food Hydrocoll. 2017, 69, 466–472. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Liu, L.; Wen, W.; Zhang, R.; Wei, Z.; Deng, Y.; Xiao, J.; Zhang, M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Guzzo, F.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Ratnasari, N.; Walters, M.; Tsopmo, A. Antioxidant and lipoxygenase activities of polyphenol extracts from oat brans treated with polysaccharide degrading enzymes. Heliyon 2017, 3, e00351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrahmany, R.; Avis, T.J.; Tsopmo, A. Treatment of oat bran with carbohydrases increases soluble phenolic acid content and influences antioxidant and antimicrobial activities. Food Res. Int. 2013, 52, 568–574. [Google Scholar] [CrossRef]
- Roberto, B.S.; Macedo, G.A.; Macedo, J.A.; Martins, I.M.; Nakajima, V.M.; Allwood, J.W.; Stewart, D.; McDougall, G.J. Immobilized tannase treatment alters polyphenolic composition in teas and their potential anti-obesity and hypoglycemic activities in vitro. Food Funct. 2016, 7, 3920–3932. [Google Scholar] [CrossRef]
- Castello, F.; Fernández-Pachón, M.-S.; Cerrillo, I.; Escudero-López, B.; Ortega, Á.; Rosi, A.; Bresciani, L.; Del Rio, D.; Mena, P. Absorption, metabolism, and excretion of orange juice (poly) phenols in humans: The effect of a controlled alcoholic fermentation. Arch. Biochem. Biophys. 2020, 695, 108627. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Changes in the Composition of Methylxanthines, Polyphenols, and Volatiles and Sensory Profiles of Cocoa Beans from the Sul 1 Genotype Affected by Fermentation. J. Agric. Food Chem. 2020, 68, 8658–8675. [Google Scholar] [CrossRef]
- Avasilcai, L.; Teliban, G.; Morariu, D.I.; Stoleru, V.; Bibire, N.; Vieriu, M.; Panainte, A.D.; Munteanu, N. Parameters of chemical composition of Phaseolus coccineus L. pods grown in protected areas. Methods 2017, 68, 2955–2958. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef] [PubMed]
- Csatlos, N.-I.; Simon, E.; Teleky, B.-E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.-C.; Ciont, C.; Pop, O.-L. Development of a Fermented Beverage with Chlorella Vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, S44–S55. [Google Scholar] [CrossRef]
- Tang, V.C.Y.; Sun, J.; Cornuz, M.; Yu, B.; Lassabliere, B. Effect of solid-state fungal fermentation on the non-volatiles content and volatiles composition of Coffea canephora (Robusta) coffee beans. Food Chem. 2021, 337, 128023. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Tlais, A.Z.A.; Morozova, K.; Cavoski, I.; Scampicchio, M.; Gobbetti, M.; Di Cagno, R. Lactic acid fermentation enriches the profile of biogenic fatty acid derivatives of avocado fruit (Persea americana Mill.). Food Chem. 2020, 317, 126384. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, P.; Zhu, Z.; Chen, C.; Xu, X. Production of phenolic compounds and antioxidant activity via bioconversion of wheat straw by Inonotus obliquus under submerged fermentation with the aid of a surfactant. J. Sci. Food Agric. 2021, 101, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Song, C.; Shi, Q.; Tian, J.; Chen, C.; Huang, J.; She, B.; Zhao, X.; Huang, R.; Jin, S. A novel predict-verify strategy for targeted metabolomics: Comparison of the curcuminoids between crude and fermented turmeric. Food Chem. 2020, 331, 127281. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, A.-J.; Park, M.-J.; Kang, K.; Soung, D.Y. Nutritional and Functional Properties of Fermented Mixed Grains by Solid-State Fermentation with Bacillus amyloliquefaciens 245. Foods 2020, 9, 1693. [Google Scholar] [CrossRef]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid state lactic acid fermentation: A strategy to improve wheat bran functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- Khan, S.A.; Zhang, M.; Liu, L.; Dong, L.; Ma, Y.; Wei, Z.; Chi, J.; Zhang, R. Co-culture submerged fermentation by lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. 2020, 326, 126985. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT-Food Sci. Tehcnol. 2020, 125, 109264. [Google Scholar] [CrossRef]
- Norakma, M.N.; Zaibunnisa, A.H.; Razarinah, W.A.R.W. The changes of phenolics profiles, amino acids and volatile compounds of fermented seaweed extracts obtained through microbial fermentation. Mater. Today Proc. 2022, 48, 815–821. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of Isoflavones after Ingestion of Soy Beverages in Healthy Adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef] [Green Version]
- Nagino, T.; Kano, M.; Masuoka, N.; Kaga, C.; Anbe, M.; Miyazaki, K.; Kamachi, K.; Isozaki, M.; Suzuki, C.; Kasuga, C. Intake of a fermented soymilk beverage containing moderate levels of isoflavone aglycones enhances bioavailability of isoflavones in healthy premenopausal Japanese women: A double-blind, placebo-controlled, single-dose, crossover trial. Biosci. Microbiota Food. Health 2016, 35, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Jang, H.-H.; Noh, H.; Kim, H.-W.; Cho, S.-Y.; Kim, H.-J.; Lee, S.-H.; Lee, S.-H.; Gunter, M.J.; Ferrari, P.; Scalbert, A. Metabolic tracking of isoflavones in soybean products and biosamples from healthy adults after fermented soybean consumption. Food Chem. 2020, 330, 127317. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and storage effect on berry polyphenols: Challenges and implications for bioactive properties. J. Agricult. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef]
- Walther, B.; Sieber, R. Bioactive proteins and peptides in foods. Int. J. Vitam. Nutr. Res. 2011, 81, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals 2021, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S. Encapsulation for Improving in Vitro Gastrointestinal Digestion of Plant Polyphenols and Their Applications in Food Products. Food Rev. Int. 2022, 38, 335–353. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Pulicharla, R.; Marques, C.; Das, R.K.; Rouissi, T.; Brar, S.K. Encapsulation and release studies of strawberry polyphenols in biodegradable chitosan nanoformulation. Int. J. Biol. Macromol. 2016, 88, 171–178. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.I.; Torres, A.; Caballero-George, C. New Curcumin-Loaded Chitosan Nanocapsules: In Vivo Evaluation. Planta Med. 2017, 83, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation optimization of lemon balm antioxidants in calcium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Wang, M.; Ji, N.; Li, M.; Li, Y.; Dai, L.; Zhou, L.; Xiong, L.; Sun, Q. Fabrication and characterization of starch beads formed by a dispersion-inverse gelation process for loading polyphenols with improved antioxidation. Food Hydrocoll. 2020, 101, 105565. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.-G.; Xiao, Z.-G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef] [PubMed]
- Santana, Á.L.; Macedo, G.A. Challenges on the processing of plant-based neuronutraceuticals and functional foods with emerging technologies: Extraction, encapsulation and therapeutic applications. Trends Food Sci. Technol. 2019, 91, 518–529. [Google Scholar] [CrossRef]
- Liu, E.; Segato, F.; Prade, R.A.; Wilkins, M.R. Exploring lignin depolymerization by a bi-enzyme system containing aryl alcohol oxidase and lignin peroxidase in aqueous biocompatible ionic liquids. Bioresour. Technol. 2021, 338, 125564. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food. Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Markgren, J.; Hedenqvist, M.; Rasheed, F.; Skepo, M.; Johansson, E. Glutenin and Gliadin, a Piece in the Puzzle of their Structural Properties in the Cell Described through Monte Carlo Simulations. Biomolecules 2020, 10, 1095. [Google Scholar] [CrossRef]


| Food | Processing Conditions | Effect of Polyphenols Bioavailability | References |
|---|---|---|---|
| Cassava | Boiling (~98 °C, 25 min) and steaming (over boiling water, (~100 °C, 25 min) | ↑ total phenolic bio-accessibility total polyphenols bio-accessibility >70% ↓ total phenolic content after the in vitro digestion, 12.10 mg GA/100 g (boiling) and 19.24 mg GA/100 g (steaming) before the in vitro digestion16.59 mg GA/100 g (boiling), 25.81 mg GA/100 g (steaming) | [65] |
| Baobab (Adansonia digitata) juice | Pasteurization 72 °C, 15 s | ↓ total phenol content significantly ↓ procyanidin B2 by 12.6% ↑ (-)-epicatechin by 10.9% | [64] |
| Orange juice | Thermal treatment (85 °C, 1–15 min; 99 °C, 10 s) | protect total polyphenol bio-accessibility | [66] |
| Black mulberry | Jam processing (95 °C, 25 min) | ↑ total polyphenol bio-accessibility ↓ flavonoids bio-accessibility ↓ total phenolics (by 88%), total flavonoids (by 89%), anthocyanins (97%), and antioxidant capacity (88–93%) ↑ recovery of bioaccessible total phenolics, ↑ anthocyanins and total antioxidant capacity (having the recovery values of 16%, 12%, and 37% for TPC, TMA, and TAC (ABTS) ↓ of bioaccessible total anthocyanins (5%) ↑ the recovery of bioaccessible total antioxidant capacity: CUPRAC (36%) | [68] |
| Mulberry juice-enriched dried minced pork slices | dried 10 h at 40 °C followed by baking 3 min at 150 °C | ↑ phenols retention rates (54.84% polyphenols, 39.1% flavonoids, and 59.62% anthocyanins) | [69] |
| Apple pomace | T = 30, 50, 80, 80, 100 and 100 °C (from feed to die) Feed rate = 30 kg/h screw speed = 370 rpm | significantly ↑ the antioxidant activity (ORAC) in the in vitro gastrointestinal digestion total extractable polyphenols, measured as gallic acid equivalents, ↓ by extrusion (barrel moisture 30%) but was not affected by extrusion at lower barrel moistures (15% or 20%) | [70] |
| Juçara (Euterpe edulis Martius)-based smoothie (juçara (20%), banana (40%) and strawberry (40%) | Pasteurization 90 °C, 35 s | ↑ total phenolic compounds bio-accessibility (47%), ferulic (16%), and ellagic (80%) acids in vitro intestinal bio-accessibility varied from 20 to 47% in vitro gastric and intestinal digestion bio-accessibility of the phenolic compounds was ↑ in the intestinal digest, due to the increase of pH ↑ more in the pasteurized smoothies than in the control sample | [71] |
| Strawberry-kale-mix (strawberry) puree 20% and kale juice 80%) | Thermal treatment (70 °C, 2 min) | ↓ free anthocyanin content after gastric digestion with 44% for PR and 48% for CG minor ↑ (8% ± 2%) for CG and 16% ± 2% for PR and ↓ by 15% to 18% in PG, PMG and PAG | [63] |
| Wheat bran (WB) and oat bran (OB) | Thermal treatment 10 min at 80 °C | ↑ total phenolic content for WB (ferulic acid + 39.18%, vanillic acid + 95.68%, apigenin–glucoside + 71.96%, p-coumaric acid + 71.91%) and of OB (avenanthramide 2c + 52.17%, dihydroxybenzoic acids + 38.55%) | [72] |
| Polyphenols | Bonding Compounds | Interactions/Process | Test/Effect/Availability | References |
|---|---|---|---|---|
| Polyphenols from black carrots (anthocyanins and phenolic acids) | Dietary lipids from coconut oil, sunflower oil, and beef tallow | Hydrophobic interactions and hydrogen bonds | simulated in vitro gastrointestinal digestion and colonic fermentation ↑ accessibility | [94] |
| Curcumin | Lipids (milk fat) | Hydrogen bond interactions | after in vitro gastrointestinal digestion, 11% of the curcuminoids delivered in yoghurt was degraded compared to <1% for curcuminoids in aqueous dispersion, but was 15-fold more bio-accessible than curcuminoids in aqueous dispersion | [95] |
| Rosmarinic acid | Whey protein (α-lactalbumin, β-lactoglobulin, and Lactoferrin) | Hydrogen and hydrophobic bonds and van der Waals interaction | ↓ rosmarinic acid activity in the presence of milk proteins ↑ in protein stability | [43,96] |
| Oat polyphenols | Casein and whey protein | Covalent interaction Hydrogen bonds | in vitro gastric and pancreatic digestion ↑ antioxidant activity and bio-accessibility of oat phenolics when mixed with milk whey protein | |
| Tea polyphenols | β-Lactoglobulin caseino-macro-peptide | Hydrophobic interactions and hydrogen bonds | maintain anti-proliferative activity against different tumour cell lines ↑ accessibility, synergetic effects | [97] |
| Green tea epigallocatechin- 3-gallate (EGCG) | Bovine α-lactalbumin (ALA) | Hydrophobic interactions | non-covalent interactions, binding affinity, and binding site between ALA and EGCG ↑ biological activity of EGCG | [98] |
| Rutin | Bovine β-lactoglobulin (BLG) | Hydrogen and hydrophobic interaction | BLG can serve as a suitable transporter for the hydrophobic ligand | [99] |
| Resveratrol | Gliadin | Colloidal complexes | in vitro gastrointestinal digestion model bio-accessibility ↑ lipid oxidation stability | [100] |
| Grape polyphenols | Cellulose–lignin hydrogels | Hydrogen bond interactions | depending on the lignin content, hydrogels can control the release of polyphenols | [101] |
| Fermentation Type | Matrix | Microorganisms | Influence on Polyphenols | Determination Method | References |
|---|---|---|---|---|---|
| Lactic | Orange juice milk-based beverage | L. brevis POM, L. plantarum TR-71, TR-14 | ↑ total polyphenol content | Spectrometry | [115] |
| Intestinal fermentation | Water-insoluble cocoa fraction | Bacteroides–Prevotella spp., Bifidobacterium genus, Lactobacillus– Enterococcus group, Clostridium histolyticum group | ↑ polyphenol content | LC/MS/MS | [116,117] |
| Solid-state fermentation | Sul 1 cacao | Ceratobasidium theobromae | ↓ polyphenol content and methylxanthines (theobromine and caffeine) | NP-HPLC RP-HPLC | [109] |
| Solid-state fermentation | Dandelion | L. plantarum (CGMCC No. 1.12934) S. cerevisiae (CGMCC No. 2.1190) | ↑ polyphenol content | UPLC-ESI-MS/MS | [113] |
| Lactic acid fermentation | Kiwifruit | L. plantarum | ↑ total phenolic content | Spectrometry | [23] |
| Solid-state fermentation | Ginger | S. cerevisiae, Bacillus licheniformis, B. pumilus, B. safensis | ↑ polyphenol content | Titration | [54] |
| Solid-state fungal fermentation | Green coffee beans | Aspergillus luchuensis Inui (JCM 22239), A. Oryzae (Ahlburg) Cohn var. Brunnues Murakami (JCM 2059), Mucor plumbeus Bonorden (JCM 3900) | ↓ polyphenol content | HPLC | [118] |
| Solid-state fermentation | Lentil cultivars | Aspergillus awamori (MTCC 548) | ↑ polyphenol content | HPLC | [119] |
| Lactic acid fermentation | Avocado fruits | L. plantarum AVEF17 | ↑ polyphenol content | Spectrometry | [120] |
| Malolactic fermentation | Sea buckthorn, Sea buckthorn-apple juice | L. plantarum, Argentoratensis, Oenococcus oeni | ↑ polyphenol content | UPLC-PDA UPLC | [121] |
| Submerged fermentation | Wheat straw | Inonotus obliquus | ↑ polyphenol content | HPLC-DAD ESI–MS/MS | [122] |
| Fungal fermentation | Turmeric | Monascus purpureus, Eurotium cristatum | ↑ polyphenol content | LC-QTOF-MS/MS | [123] |
| Solid-state fermentation | Mixed grains | Bacillus amyloliquefaciens 245 | ↑ polyphenol content | Spectrometry CE-TOF-MS | [124] |
| Solid-state lactic acid fermentation | Wheat bran | L. rhamnosus | ↓ total phenolic content slightly ↑ polyphenol content | Spectrometry | [125] |
| Controlled alcoholic fermentation | Orange juice | Saccharomycetaceae Pichia kluyveri | ↑ polyphenol content | UHPLC | [108] |
| Co-culture submerged fermentation | Extruded brown rice | L. plantarum, L. fermentum, Saccharomyces cerevisiae | ↑ total polyphenol content | Spectrometry | [126] |
| Solid-state fermentation | Whole soybean flour | L. casei | ↑ total polyphenol content | HPLC | [127] |
| Solid-state fermentation | Seaweed | Aspergillus oryzae | ↑ polyphenol content | LC-MS/MS | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, O.L.; Suharoschi, R.; Socaci, S.A.; Berger Ceresino, E.; Weber, A.; Gruber-Traub, C.; Vodnar, D.C.; Fărcaș, A.C.; Johansson, E. Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments. Antioxidants 2023, 12, 865. https://doi.org/10.3390/antiox12040865
Pop OL, Suharoschi R, Socaci SA, Berger Ceresino E, Weber A, Gruber-Traub C, Vodnar DC, Fărcaș AC, Johansson E. Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments. Antioxidants. 2023; 12(4):865. https://doi.org/10.3390/antiox12040865
Chicago/Turabian StylePop, Oana Lelia, Ramona Suharoschi, Sonia Ancuța Socaci, Elaine Berger Ceresino, Achim Weber, Carmen Gruber-Traub, Dan Cristian Vodnar, Anca Corina Fărcaș, and Eva Johansson. 2023. "Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments" Antioxidants 12, no. 4: 865. https://doi.org/10.3390/antiox12040865
APA StylePop, O. L., Suharoschi, R., Socaci, S. A., Berger Ceresino, E., Weber, A., Gruber-Traub, C., Vodnar, D. C., Fărcaș, A. C., & Johansson, E. (2023). Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments. Antioxidants, 12(4), 865. https://doi.org/10.3390/antiox12040865