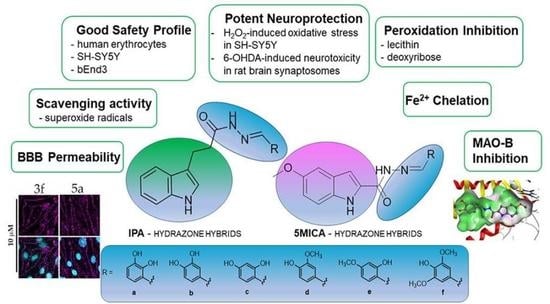
New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Synthesis of Compounds 1, 2 and 4
2.3. General Procedure for the Synthesis of Compounds 3a–f and 5a–f
2.3.1. N′-(2,3-Dihydroxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3a)
2.3.2. N′-(3,4-Dihydroxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3b)
2.3.3. N′-(2,4-Dihydroxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3c)
2.3.4. N′-(4-Hydroxy-3-methoxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3d)
2.3.5. N′-(4-Hydroxy-2-methoxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3e)
2.3.6. N′-(4-Hydroxy-3,5-dimethoxybenzylidene)-3-(1H-indol-3-yl)propanehydrazide (3f)
2.3.7. N′-(2,3-Dihydroxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5a)
2.3.8. N′-(3,4-Dihydroxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5b)
2.3.9. N′-(2,4-Dihydroxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5c)
2.3.10. N′-(4-Hydroxy-3-methoxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5d)
2.3.11. N′-(2-Hydroxy-4-methoxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5e)
2.3.12. N′-(4-Hydroxy-3,5-dimethoxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide (5f)
2.4. Red Blood Cell Hemolysis Assay
2.5. Cell lines and Culturing Conditions
2.6. In Vitro Cell Viability Assay
2.7. H2O2-Induced Oxidative Stress Model in SH-SY5Y Cells
2.8. Ferrous Iron-Induced Oxidative Damage
2.9. Lipid Peroxidation Assay (LP Assay)
2.10. Deoxyribose Degradation Assay
2.11. O-Phenanthroline Test
2.12. Superoxide Radicals Scavenging Activity
2.13. Permeability Assay
2.14. Immunofluorescence
2.15. hMAO-B Enzyme Activity Inhibition
2.16. Animals
2.17. Isolation of the Rat-Brain Synaptosomes
2.18. Measurement of Synaptosomal Viability
2.19. Level of Reduced Glutathione (GSH) in Isolated Synaptosomes
2.20. Model of 6-Hydroxy Dopamine-Induced Neurotoxicity in Isolated Rat Synaptosomes
2.21. Statistical Analysis
2.22. Molecular Docking
3. Results
3.1. Synthesis of the Target Compounds
3.2. Safety Profile
3.3. Neuroprotective Effects in H2O2-Induced Oxidative Stress on SH-SY5Y Cells and 6-OHDA-Induced Neurotoxicity in Rat-Brain Synaptosomes
3.4. Iron-Induced Oxidative Damage of Lecithin and Deoxyribose
3.5. Ortho-Phenanthroline Test
3.6. Superoxide Radicals Scavenging Activity
3.7. hMAO-B enzyme Inhibition
3.8. BBB Model Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Angelova, P. Sources and triggers of oxidative damage in neurodegeneration. Free Radic. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Significance of Antioxidants on Aging and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 13957. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carayon, A.; Javoy-agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991, 114 Pt 4, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.M.; Du, G.; Kidacki, M.; Patel, N.; Shaffer, M.L.; Mailman, R.B.; Huang, X. Higher iron in the red nucleus marks Parkinson’s dyskinesia. Neurobiol. Aging 2013, 34, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chen, C.M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef]
- Palanimuthu, D.; Wu, Z.; Jansson, P.J.; Braidy, N.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer’s disease. Dalton Trans. 2018, 47, 7190–7205. [Google Scholar] [CrossRef]
- Wu, Z.; Palanimuthu, D.; Braidy, N.; Salikin, N.H.; Egan, S.; Huang, M.L.H.; Richardson, D.R. Novel multifunctional iron chelators of the aroyl nicotinoyl hydrazone class that markedly enhance cellular NAD(+)/NADH ratios. Br. J. Pharmacol. 2020, 177, 1967–1987. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Jeong, Y.G.; Lee, Y.L.; Kim, Y.M.; Kwon, Y.G.; et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J. Neurosci. Res. 2009, 87, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Bendheim, P.E.; Poeggeler, B.; Neria, E.; Ziv, V.; Pappolla, M.A.; Chain, D.G. Development of indole-3-propionic acid (OXIGON) for Alzheimer’s disease. J. Mol. Neurosci. 2002, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Ivanova, N.; Nenchovska, Z.; Tzoneva, R.; Stoyanova, T.; Uzunova, V.; Surcheva, S.; Tzonev, A.; Angelova, V.T.; Andreeva-Gateva, P. Evaluation of neurobiological and antioxidant effects of novel melatonin analogues in mice. Saudi Pharmaceut. J. 2020, 28, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Stoyanova, T.; Tzoneva, R.; Angelova, V.; Andreeva-Gateva, P. The Anticonvulsant Effect of a Novel Indole-Related Compound in the Kainate-Induced Status Epilepticus in Mice: The Role of the Antioxidant and Anti-inflammatory Mechanism. Neurochem. Res. 2022, 47, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.J.; Poeggeler, B.; Omar, R.A.; Chain, D.G.; Frangione, B.; Ghiso, J.; Pappolla, M.A. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem. 1999, 274, 21937–21942. [Google Scholar] [CrossRef]
- Tzoneva, R.; Georgieva, I.; Ivanova, N.; Uzunova, V.; Nenchovska, Z.; Apostolova, S.; Stoyanova, T.; Tchekalarova, J. The Role of melatonin on behavioural changes and concomitant oxidative stress in icvAβ1-42 rat model with pinealectomy. Int. J. Mol. Sci. 2021, 22, 12763. [Google Scholar] [CrossRef]
- Sangchart, P.; Panyatip, P.; Damrongrungruang, T.; Priprem, A.; Mahakunakorn, P.; Puthongking, P. Anti-Inflammatory Comparison of Melatonin and Its Bromobenzoylamide Derivatives in Lipopolysaccharide (LPS)-Induced RAW 264.7 Cells and Croton Oil-Induced Mice Ear Edema. Molecules 2021, 26, 4285. [Google Scholar] [CrossRef]
- Wei, P.C.; Lee-Chen, G.J.; Chen, C.M.; Wu, Y.R.; Chen, Y.J.; Lin, J.L.; Lo, Y.S.; Yao, C.F.; Chang, K.H. Neuroprotection of Indole-Derivative Compound NC001-8 by the Regulation of the NRF2 Pathway in Parkinson’s Disease Cell Models. Oxid. Med. Cell Longev. 2019, 2019, 5074367. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Lin, C.-H.; Lin, C.-Y.; Yang, P.-N.; Lo, Y.-S.; Chen, Y.-C.; Chen, C.-M.; Wu, Y.-R.; Yao, C.-F.; Chang, K.-H.; et al. Investigating Therapeutic Effects of Indole Derivatives Targeting Inflammation and Oxidative Stress in Neurotoxin-Induced Cell and Mouse Models of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 2642. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Fu, Y.; Zhang, L.; Gong, J.-X.; Wang, Z.-Z.; Xiao, W.; Guo, Y.-W. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg. Med. Chem. Lett. 2015, 25, 216–220. [Google Scholar] [CrossRef]
- Prins, L.H.; Petzer, J.P.; Malan, S.F. Inhibition of monoamine oxidase by indole and benzofuran derivatives. Eur. J. Med. Chem. 2010, 45, 4458–4466. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; de Candia, M.; De Palma, A.; De Santis, F.; Pisani, L.; Campagna, F.; Cellamare, S.; Altomare, C.D.; Catto, M. Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules 2018, 23, 1544. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, W.; Chavan, P.C.; Hanamshetty, N.M.M.; Thippeswamy, A. Biological Importance Of The Indole Nucleus In Re-Cent Years: A Comprehensive Review. Int. J. Curr. Pharmaceut. Rev. Res. 2022, 14, 46–63. [Google Scholar]
- Mahmoud, M.M.; Ali, H.I.; Ahn, K.H.; Damaraju, A.; Samala, S.; Pulipati, V.K.; Kolluru, S.; Kendall, D.A.; Lu, D. Structure-activity relationship study of indole-2-carboxamides identifies a potent allo-steric modulator for the cannabinoid receptor 1 (CB1). J. Med. Chem. 2013, 56, 7965–7975. [Google Scholar] [CrossRef] [PubMed]
- Bowroju, S.K.; Mainali, N.; Ayyadevara, S.; Penthala, N.R.; Krishnamachari, S.; Kakraba, S.; Reis, R.J.S. Crooks PA. Design and Synthesis of Novel Hybrid 8-Hydroxy Quinoline-Indole Derivatives as Inhibitors of Aβ Self-Aggregation and Metal Chelation-Induced Aβ Aggregation. Molecules 2020, 25, 3610. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Ponka, P. Pyridoxal isonicotinoyl hydrazone and its analogues: Potential orally effective iron-chelating agents for the treatment of iron overload disease. J. Lab. Clin. Med. 1998, 131, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, D.S.; Rey, N.A. Tridentate N-Acylhydrazones as Moderate Ligands for the Potential Management of Cognitive Decline Associated With Metal-Enhanced Neuroaggregopathies. Front. Neurol. 2022, 13, 828654. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, D.S.; Pinheiro, A.B.; Castiñeiras-Filho, S.L.; da Silva, A.S.; Miotto, M.C.; De Falco, A.; de P. Ribeiro, T.; Maisonette, S.; da Cunha, A.L.M.C.; Hauser-Davis, R.A.; et al. A moderate metal-binding hydrazone meets the criteria for a bioinorganic approach towards Parkinson’s disease: Therapeutic potential, blood-brain barrier crossing evaluation and preliminary toxicological studies. J. Inorg. Biochem. 2017, 170, 160–168. [Google Scholar] [CrossRef]
- Carradori, S.; Ortuso, F.; Petzer, A.; Bagetta, D.; De Monte, C.; Secci, D.; De Vita, D.; Guglielmi, P.; Zengin, G.; Aktumsek, A.; et al. Design, synthesis and biochemical evaluation of novel multi-target inhibitors as potential anti-Parkinson agents. Eur. J. Med. Chem. 2018, 143, 1543–1552. [Google Scholar] [CrossRef]
- D’Ascenzio, M.; Chimenti, P.; Gidaro, M.C.; De Monte, C.; De Vita, D.; Granese, A.; Scipione, L.; Di Santo, R.; Costa, G.; Alcaro, S.; et al. Thiazol-2-yl)hydrazone derivatives from acetylpyridines as dual inhibitors of MAO and AChE: Synthesis, biological evaluation and molecular modeling studies. J. Enzyme Inhib. Med. Chem. 2015, 30, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Anastassova, N.; Aluani, D.; Hristova-Avakumova, N.; Tzankova, V.; Kondeva-Burdina, M.; Rangelov, M.; Todorova, N.; Yancheva, D. Study on the Neuroprotective, Radical-Scavenging and MAO-B Inhibiting Properties of New Benzimidazole Arylhydrazones as Potential Multi-Target Drugs for the Treatment of Parkinson’s Disease. Antioxidants 2022, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Anastassova, N.; Aluani, D.; Kostadinov, A.; Rangelov, M.; Todorova, N.; Hristova-Avakumova, N.; Argirova, M.; Lumov, N.; Kondeva-Burdina, M.; Tzankova, V.; et al. Evaluation of the combined activity of benzimidazole arylhydrazones as new anti-Parkinsonian agents: Monoamine oxidase-B inhibition, neuroprotection and oxidative stress modulation. Neural. Regen. Res. 2021, 16, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Anastassova, N.; Yancheva, D.; Hristova-Avakumova, N.; Hadjimitova, V.; Traykov, T.; Aluani, D.; Tzankova, V.; Kondeva-Burdina, M. New benzimidazol-aldehyde hybrids as neuroprotectors with hypochlorite and super oxide radical scavenging activity. Pharmacol. Rep. 2020, 72, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of pH-responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs. J. Vis. Exp. 2013, 73, 50166. [Google Scholar] [CrossRef]
- ISO—ISO 10993-5:2009—Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 23 January 2023).
- Montesano, R.; Pepper, M.S.; Möhle-Steinlein, U.; Risau, W.; Wagner, E.F.; Orci, L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 1990, 62, 435–445. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 11059. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T. Oxidative stability of egg and soy lecithin as affected by transition metal ions and pH in emulsion. J. Agric. Food Chem. 2008, 56, 11424–11431. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Modification of the deoxyribose test to detect strong iron binding. Acta Biochim. Pol. 2017, 64, 195–198. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration, and Spectrophotometry in Inorganic Analysis; Elsevier: New York, NY, USA, 2000; Volume 10, pp. 3–521. [Google Scholar]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H. Determination of Xanthine Oxidase. Pharm. Anal. Acta S 2013, S7, 004. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mutterer, J.; Zinck, E. Quick-and-clean article figures with FigureJ. J. Microsc. 2013, 252, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kasabova-Angelova, A.; Kondeva-Burdina, M.; Mitkov, J.; Georgieva, M.; Tzankova, V.; Zlatkov, A. Neuroprotective and MAOB inhibitory effects of a series of caffeine-8-thioglycolic acid amides. Brazilian J. Pharm. Sci. 2020, 56, 18255. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Esteban, G.; Bolea, I.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Samadi, A.; Soriano, E.; Unzeta, M.; et al. Design, synthesis, pharmacological evaluation, QSAR analysis, molecular modeling and ADMET of novel donepezil-indolyl hybrids as multipotent cholinesterase/monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Held, P. Quantitation of Hydrogen Peroxide Using the Synergy HT. In Application; BioTek: Santa Clara, CA, USA, 2003; p. 6. [Google Scholar]
- Taupin, P.; Zini, S.; Cesselin, F.; Ben-Ari, Y.; Roisin, M.-P. Subcellular Fractionation on Percoll Gradient of Mossy Fiber Synaptosomes: Morphological and Biochemical Characterization in Control and Degranulated Rat Hippocampus. J. Neurochem. 1994, 62, 1586–1595. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mungarro-Menchaca, X.; Morán, P.F.J.; Clorinda, A. β-Amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J. Neurosci. Res. 2002, 68, 89–96. [Google Scholar] [CrossRef]
- Robyt, J.F.; Ackerman, R.J.; Chittenden, C.G. Reaction of protein disulfide groups with Ellman’s reagent: A case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae α-amylase, papain, and lysozyme. Arch. Biochem. Biophys. 1971, 147, 262–269. [Google Scholar] [CrossRef]
- Stokes, A.H.; Freeman, W.M.; Mitchell, S.G.; Burnette, T.A.; Hellmann, G.M.; Vrana, K.E. Induction of GADD45 and GADD153 in Neuroblastoma Cells by Dopamine-Induced Toxicity. Neurotoxicology 2002, 23, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Aldeco, M.; Mattevi, A.; Edmondson, D.E. Interactions of monoamine oxidases with the antiepileptic drug zonisamide: Specificity of inhibition and structure of the human monoamine oxidase B complex. J. Med. Chem. 2011, 54, 909–912. [Google Scholar] [CrossRef] [PubMed]
- H3A 2R7; Molecular Operating Environment (MOE). Chemical Computing Group Inc.: Montreal, QC, Canada, 2020.
- Sowemimo-Coker, S.O. Red blood cell hemolysis during processing. Transfus. Med. Rev. 2002, 16, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Robaszkiewicz, A.; Balcerczyk, A.; Bartosz, G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007, 31, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Branen, A.L. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975, 52, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol. 1994, 233, 57–66. [Google Scholar] [CrossRef]
- Chobot, V. Simultaneous Detection of Pro- and Antioxidative Effects in the Variants of the Deoxyribose Degradation Assay. J. Agric. Food Chem. 2010, 58, 2088. [Google Scholar] [CrossRef]
- Rachmilovich-Calis, S.; Meyerstein, N.; Meyerstein, D. A Mechanistic Study of the Effects of Antioxidants on the Formation of Malondialdehyde-Like Products in the Reaction of Hydroxyl Radicals with Deoxyribose. Chem.–A Eur. J. 2009, 15, 7717–7723. [Google Scholar] [CrossRef]
- Binda, C.; Newton-Vinson, P.; Hubalek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002, 9, 22–26. [Google Scholar] [CrossRef]
- Edmondson, D.E.; Binda, C.; Wang, J.; Upadhyay, A.K.; Mattevi, A. Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry 2009, 48, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Esteban, G.; Allan, J.; Samadi, A.; Mattevi, A.; Unzeta, M.; Marco-Contelles, J.; Binda, C.; Ramsay, R.R. Kinetic and structural analysis of the irreversible inhibition of human monoamine oxidases by ASS234, a multi-target compound designed for use in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1844, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Manzella, N.; Cagide, F.; Mialet-Perez, J.; Uriarte, E.; Parini, A.; Borges, F.; Binda, C. Tight-Binding Inhibition of Human Monoamine Oxidase B by Chromone Analogs: A Kinetic, Crystallographic, and Biological Analysis. J. Med. Chem. 2018, 61, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Al Awabdh, S.; Coumoul, X.; Chauvet, C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Santerre, M.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2021, 2311, 9–23. [Google Scholar] [CrossRef]
- Bell, M.; Zempel, H. SH-SY5Y-derived neurons: A human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability. Rev. Neurosci. 2021, 33, 1–15. [Google Scholar] [CrossRef]
- Segura-Collar, B.; Mata-Martínez, P.; Hernández-Laín, A.; Sánchez-Gómez, P.; Gargini, R. Blood-Brain Barrier Disruption: A Common Driver of Central Nervous System Diseases. Neuroscientist 2022, 28, 222–237. [Google Scholar] [CrossRef]
- Watanabe, T.; Dohgu, S.; Takata, F.; Nishioku, T.; Nakashima, A.; Futagami, K.; Yamauchi, A.; Kataoka, Y. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol. Pharm. Bull. 2013, 36, 492–495. [Google Scholar] [CrossRef]
- Tehrani, S.F.; Bernard-Patrzynski, F.; Puscas, I.; Leclair, G.; Hildgen, P.; Roullin, V.G. Length of surface PEG modulates nanocarrier transcytosis across brain vascular endothelial cells. Nanomedicine 2019, 16, 185–194. [Google Scholar] [CrossRef]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef]
- Rahman, N.A.; Rasil, A.N.; Ain, H.M.; Meyding-Lamade, U.; Craemer, E.M.; Diah, S.; Tuah, A.A.; Muharram, S.H. Immortalized endothelial cell lines for in vitro blood-brain barrier models: A systematic review. Brain Res. 2016, 1642, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, Y.; Qian, Z.; Shen, X. The mechanism of Fe(2+)-initiated lipid peroxidation in liposomes: The dual function of ferrous ions, the roles of the pre-existing lipid peroxides and the lipid peroxyl radical. Biochem. J. 2000, 352 Pt 1, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M.; Filograna, R.; Beltramini, M.; Bubacco, L. Are dopamine derivatives implicated in the pathogenesis of Parkinson’s disease? Ageing Res. Rev. 2014, 13, 107–114. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Teo, K.C.; Ho, S.L. Monoamine oxidase-B (MAO-B) inhibitors: Implications for disease-modification in Parkinson’s disease. Transl. Neurodegener. 2013, 2, 19. [Google Scholar] [CrossRef]
- Nicotra, A.; Pierucci, F.; Parvez, H.; Senatori, O. Monoamine Oxidase Expression During Development and Aging. Neurotoxicology 2004, 25, 155–165. [Google Scholar] [CrossRef]
- Santin, Y.; Resta, J.; Parini, A.; Mialet-Perez, J. Monoamine oxidases in age-associated diseases: New perspectives for old enzymes. Ageing Res. Rev. 2021, 66, 101256. [Google Scholar] [CrossRef]
- Goldstein, D.S. The catecholaldehyde hypothesis: Where MAO fits in. J. Neural Transm. 2019, 127, 169–177. [Google Scholar] [CrossRef]
- Chamoli, M.; Chinta, S.J.; Andersen, J.K. An inducible MAO-B mouse model of Parkinson’s disease: A tool towards better understanding basic disease mechanisms and developing novel therapeutics. J. Neural Transm. 2018, 125, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Pienaar, I.S. Disruption of the blood-brain barrier in parkinson’s disease: Curse or route to a cure? Front. Biosci. 2014, 19, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Devel. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Gealageas, R.; Devineau, A.; So, P.P.L.; Kim, C.M.J.; Surendradoss, J.; Buchwalder, C.; Heller, M.; Goebeler, V.; Dullaghan, E.M.; Grierson, D.S.; et al. Development of Novel Monoamine Oxidase-B (MAO-B) Inhibitors with Reduced Blood-Brain Barrier Permeability for the Potential Management of Noncentral Nervous System (CNS) Diseases. J. Med. Chem. 2018, 61, 7043–7064. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.; Ravid, O.; Atrakchi, D.; Israelov, H.; Bresler, Y.; Shemesh, C.; Omesi, L.; Liraz-Zaltsman, S.; Gosselet, F.; Maskrey, T.S.; et al. Endothelial Iron Homeostasis Regulates Blood-Brain Barrier Integrity via the HIF2α-Ve-Cadherin Pathway. Pharmaceutics 2021, 13, 311. [Google Scholar] [CrossRef]














| Compound | SH-SY5Y Cells | bEnd3 Cells | ||
|---|---|---|---|---|
| IC50 (µM) | 95% Confidence Intervals | IC50 (µM) | 95% Confidence Intervals | |
| IPA | >500 | NA | NA | |
| 3a | 119.6 | 80.2 to 140.2 | 355.6 | 329.0 to 385.1 |
| 3b | 265.3 | 212.3 to 289.2 | 352.2 | 305.6 to 405.6 |
| 3c | 275.2 | 248.2 to 311.3 | >500 | |
| 3d | 155.9 | 115.7 to 219.1 | 367.3 | 361.4 to 373.5 |
| 3e | >500 | 85.5 | 75.6 to 96.6 | |
| 3f | >500 | >500 | ||
| 5a | 79.3 | 59.63 to 105.6 | 197.4 | 169.0 to 230.6 |
| 5b | 111.1 | 95.31 to 128.8 | 157.9 | 131.6 to 189.6 |
| 5c | 73.9 | 56.3 to 89.32 | >500 | |
| 5d | 98.2 | 79.4 to 111.5 | 352.8 | 325.4 to 382.6 |
| 5e | 199.5 | 187.42 to 204.54 | 12.1 | 9.3 to 15.5 |
| 5f | 286.9 | 235.3 to 320.1 | 184.0 | 145.9 to 232.1 |
| Melatonin | >500 | >500 | ||
| Rasagiline.HCl | >500 | NA | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastassova, N.; Stefanova, D.; Hristova-Avakumova, N.; Georgieva, I.; Kondeva-Burdina, M.; Rangelov, M.; Todorova, N.; Tzoneva, R.; Yancheva, D. New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors. Antioxidants 2023, 12, 977. https://doi.org/10.3390/antiox12040977
Anastassova N, Stefanova D, Hristova-Avakumova N, Georgieva I, Kondeva-Burdina M, Rangelov M, Todorova N, Tzoneva R, Yancheva D. New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors. Antioxidants. 2023; 12(4):977. https://doi.org/10.3390/antiox12040977
Chicago/Turabian StyleAnastassova, Neda, Denitsa Stefanova, Nadya Hristova-Avakumova, Irina Georgieva, Magdalena Kondeva-Burdina, Miroslav Rangelov, Nadezhda Todorova, Rumiana Tzoneva, and Denitsa Yancheva. 2023. "New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors" Antioxidants 12, no. 4: 977. https://doi.org/10.3390/antiox12040977
APA StyleAnastassova, N., Stefanova, D., Hristova-Avakumova, N., Georgieva, I., Kondeva-Burdina, M., Rangelov, M., Todorova, N., Tzoneva, R., & Yancheva, D. (2023). New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors. Antioxidants, 12(4), 977. https://doi.org/10.3390/antiox12040977