Cydonia oblonga Mill. Pulp Callus Inhibits Oxidative Stress and Inflammation in Injured Cells
Abstract
:1. Introduction
2. Materials and Methods
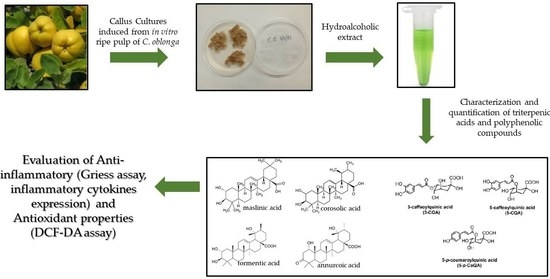
2.1. Callus Production
2.2. Callus Extract Preparation
2.3. Chemical Characterization of SMs in Quince Callus Extract
2.4. Cell Culture
2.5. Cell Viability Assay WST8
2.6. Nitric Oxide Detection
2.7. LPS-Induced Inflammation in HaCaT Cells
2.8. Quantitative Real-Time PCR
2.9. Quantification of Intracellular ROS Production
2.10. Statistical Analysis
3. Results
3.1. Effects of Co ext. on Cell Viability
3.2. Anti-Inflammatory Activity of Co ext.
3.3. Effects of Co ext. on Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashraf, M.U.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. C. oblonga M., A Medicinal Plant Rich in Phytonutrients for Pharmaceuticals. Front. Pharmacol. 2016, 7, 163. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic Profile of Quince Fruit (C. oblonga Miller) (Pulp and Peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef] [PubMed]
- Bati, A.Y.E.; Kocaman, B.; Karaca Öner, E.; Açikgöz, M.A. Effects of elicitors on secondary metabolite (SM) production and antioxidant activity in sweet basil (Ocimum basilicum L.) cell suspension cultures. Not. Sci. Biol. 2022, 14, 11246. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- De Bellis, R.; Chiarantini, L.; Potenza, L.; Gorassini, A.; Verardo, G.; De Marco, R.; Benayada, L.; Stocchi, V.; Albertini, M.C.; Fraternale, D. High Production of Secondary Metabolites and Biological Activities of C. oblonga Mill. Pulp Fruit Callus. J. Funct. Foods 2022, 94, 105133. [Google Scholar] [CrossRef]
- Potenza, L.; Minutelli, M.; Stocchi, V.; Fraternale, D. Biological Potential of an Ethanolic Extract from “Mela Rosa Marchigiana” Pulp Callus Culture. J. Funct. Foods 2020, 75, 104269. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to Detect Nitric Oxide and Its Metabolites in Biological Samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Takuathung, M.N.; Potikanond, S.; Sookkhee, S.; Mungkornasawakul, P.; Jearanaikulvanich, T.; Chinda, K.; Wikan, N.; Nimlamool, W. Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed. Pharmacother. 2021, 143, 112229. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef]
- Thakur, M.; Karuna, S.; Renu, K. Phytochemicals. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–361. [Google Scholar]
- Magrone, T.; Perez de Heredia, F.; Jirillo, E.; Morabito, G.; Marcos, A.; Serafini, M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can. J. Physiol. Pharmacol. 2013, 91, 387–396. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Verardo, G.; Gorassini, A.; Ricci, D.; Fraternale, D. High Triterpenic Acids Production in Callus Cultures from Fruit Pulp of Two Apple Varieties. Phytochem. Anal. 2017, 28, 5–15. [Google Scholar] [CrossRef]
- Verardo, G.; Gorassini, A.; Fraternale, D. New Triterpenic Acids Produced in Callus Culture from Fruit Pulp of Acca Sellowiana (O. Berg) Burret. Food Res. Int. 2019, 119, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Urquiza-López, A.; Álvarez-Rivera, G.; Ballesteros-Vivas, D.; Cifuentes, A.; Del Villar-Martínez, A.A. Metabolite Profiling of Rosemary Cell Lines with Antiproliferative Potential against Human HT-29 Colon Cancer Cells. Plant Foods Hum. Nutr. 2021, 76, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Dall’Acqua, S.; Poloniato, G.; Maggi, F.; Malagoli, M. Preliminary Evaluation of Quince (C. oblonga Mill.) Fruit as Extraction Source of Antioxidant Phytoconstituents for Nutraceutical and Functional Food Applications. J. Sci. Food Agric. 2019, 99, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Oota, S. Apple flesh tissue culture and anthocyanin formation in the derived callus tissue. J. Jpn. Soc. Hortic. Sci. 1983, 52, 117–122. [Google Scholar] [CrossRef]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Saber, F.R.; Montesano, D.; Lucini, L. The UHPLC-QTOF-MS phenolic profiling and activity of Cydonia oblonga Mill. reveals a promising nutraceutical potential. Foods 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmianski, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, J.; Zhang, Q.; Chen, L.H.; Wang, Q. Corosolic Acid Ameliorates Atherosclerosis in Apolipoprotein E-Deficient Mice by Regulating the Nuclear Factor-ΚB Signaling Pathway and Inhibiting Monocyte Chemoattractant Protein-1 Expression. Circ. J. 2012, 76, 995–1003. [Google Scholar] [CrossRef]
- Baba, K.; Hiramatsu, R.; Suradej, B.; Tanigaki, R.; Koeda, S.; Waku, T.; Kataoka, T. Intracellular Trafficking and N-Linked Glycosylation of Intercellular Adhesion Molecule-1. Biol. Pharm. Bull. 2018, 41, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-Inflammatory Effect of Pomegranate Flower in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Donfack, A.R.; Tane, P.; McGaw, L.J.; Eloff, J.N. Inhibition of Nitric Oxide Production in LPS-Stimulated RAW 264.7 Macrophages and 15-LOX Activity by Anthraquinones from Pentas schimperi. Planta Med. 2016, 82, 1246–1251. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, L.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaTCells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/RepairResponse of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Rebey, I.B.; Kefi, S.; Bourgou, S.; Ouerghemmi, I.; Ksouri, R.; Tounsi, M.S.; Marzouk, B. Ripening Stage and Extraction Method Effects on Physical Properties, Polyphenol Composition and Antioxidant Activities of Cumin (Cuminum Cyminum L.) Seeds. Plant Foods Hum. Nutr. 2014, 69, 358–364. [Google Scholar] [CrossRef]
- Cassini-Vieira, P.; Araújo, F.A.; da Costa Dias, F.L.; Russo, R.C.; Andrade, S.P.; Teixeira, M.M.; Barcelos, L.S. INOS Activity Modulates Inflammation, Angiogenesis, and Tissue Fibrosis in Polyether-Polyurethane Synthetic Implants. Mediat. Inflamm. 2015, 2015, 138461. [Google Scholar] [CrossRef]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 724–728. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial By-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef] [PubMed]




| Sequence | Accession Number | |
|---|---|---|
| iNOS | F-5′-TGACCATCATGGACCACCAC-3′ R-5′-ACCAGCCAAATCCAGTCTGC-3′ | NM_000625.4 |
| IL6 | F-5′-GGTACATCCTCGACGGCATCT-3′ R-5′-GTGCCTCTTTGCTGCTTTCAC-3′ | XM_005249745.6 |
| IL-1β | F-5-AAAGAAGAAGATGGAAAAGCGATT-3′ R-5′-GGGAACTGGGCAGACTCAAATTC-3′ | XM_047444175 |
| IkBα | F-5′-GCTGCTGATGTCAATGCTCA-3′ R-5′-ACACCAGGTCAGGATTTTGC-3′ | NM_020529.3 |
| ICAM | F-5′-CCTTCCTCACCGTGTACTGG-3′ R-5′-AGCGTAGGGTAAGGTTCTTGC-3′ | NM_000201 |
| 36B4 | F 5′-CGACCTGGAAGTCCAACTAC-3′ R 5′- ATCTGCTGCATCTGCTTG-3′ | NM_053275.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubitosa, F.; Fraternale, D.; De Bellis, R.; Gorassini, A.; Benayada, L.; Chiarantini, L.; Albertini, M.C.; Potenza, L. Cydonia oblonga Mill. Pulp Callus Inhibits Oxidative Stress and Inflammation in Injured Cells. Antioxidants 2023, 12, 1076. https://doi.org/10.3390/antiox12051076
Gubitosa F, Fraternale D, De Bellis R, Gorassini A, Benayada L, Chiarantini L, Albertini MC, Potenza L. Cydonia oblonga Mill. Pulp Callus Inhibits Oxidative Stress and Inflammation in Injured Cells. Antioxidants. 2023; 12(5):1076. https://doi.org/10.3390/antiox12051076
Chicago/Turabian StyleGubitosa, Federica, Daniele Fraternale, Roberta De Bellis, Andrea Gorassini, Leila Benayada, Laura Chiarantini, Maria Cristina Albertini, and Lucia Potenza. 2023. "Cydonia oblonga Mill. Pulp Callus Inhibits Oxidative Stress and Inflammation in Injured Cells" Antioxidants 12, no. 5: 1076. https://doi.org/10.3390/antiox12051076
APA StyleGubitosa, F., Fraternale, D., De Bellis, R., Gorassini, A., Benayada, L., Chiarantini, L., Albertini, M. C., & Potenza, L. (2023). Cydonia oblonga Mill. Pulp Callus Inhibits Oxidative Stress and Inflammation in Injured Cells. Antioxidants, 12(5), 1076. https://doi.org/10.3390/antiox12051076