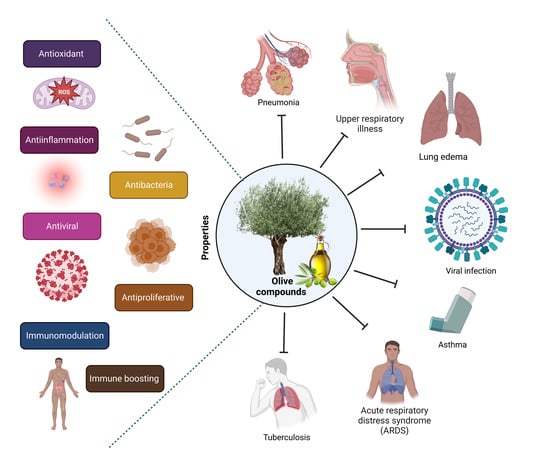
Role of Olive Bioactive Compounds in Respiratory Diseases
Abstract
:1. Introduction
2. Olive Bioactive Compounds
3. Respiratory Diseases
4. Olive Bioactive Molecules in Respiratory Inflammation
5. Olive Bioactive Molecules in Respiratory Oxidative Stress
6. Olive Bioactive Molecules in Infectious Respiratory Diseases
7. Olive Bioactive Molecules in Over-Proliferation of Respiratory Cells
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar] [CrossRef]
- Baptista, E.A.; Dey, S.; Pal, S. Chronic respiratory disease mortality and its associated factors in selected Asian countries: Evidence from panel error correction model. BMC Public. Health 2021, 21, 53. [Google Scholar] [CrossRef]
- Levine, S.M.; Marciniuk, D.D. Global Impact of Respiratory Disease: What Can We Do, Together, to Make a Difference? Chest 2022, 161, 1153–1154. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 21 March 2023).
- Geddes, D. The history of respiratory disease management. Medicine 2020, 48, 239–243. [Google Scholar] [CrossRef]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of Current Therapeutics and Novel Candidates Against Influenza, Respiratory Syncytial Virus, and Middle East Respiratory Syndrome Coronavirus Infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef]
- He, H.; Wunderink, R.G. Staphylococcus aureus Pneumonia in the Community. Semin. Respir. Crit. Care Med. 2020, 41, 470–479. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug. Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Sanders, M. Inhalation therapy: An historical review. Prim. Care Respir. J. 2007, 16, 71–81. [Google Scholar] [CrossRef]
- Oriola, A.O.; Oyedeji, A.O. Plant-Derived Natural Products as Lead Agents against Common Respiratory Diseases. Molecules 2022, 27, 3054. [Google Scholar] [CrossRef]
- Garcia-Martinez, O.; Ruiz, C.; Gutierrez-Ibanez, A.; Illescas-Montes, R.; Melguizo-Rodriguez, L. Benefits of Olive Oil Phenolic Compounds in Disease Prevention. Endocr. Metab. Immune Disord. Drug. Targets 2018, 18, 333–340. [Google Scholar] [CrossRef]
- Barazani, O.; Dag, A.; Dunseth, Z. The history of olive cultivation in the southern Levant. Front. Plant. Sci. 2023, 14, 1131557. [Google Scholar] [CrossRef]
- Letendre, D. Les mots de l’insoumis. Liberte 2014, 47. Available online: https://scholar.google.com/scholar_lookup?title=Les+mots+de+l%E2%80%99insoumis&author=Letendre,+D.&publication_year=2014&journal=Liberte&volume=47&pages=278 (accessed on 26 March 2023).
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef]
- Che Man, R.; Sulaiman, N.; Ishak, M.F.; Bt Hj Idrus, R.; Abdul Rahman, M.R.; Yazid, M.D. The Effects of Pro-Inflammatory and Anti-Inflammatory Agents for the Suppression of Intimal Hyperplasia: An Evidence-Based Review. Int. J. Environ. Res. Public Health 2020, 17, 7825. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Yazid, M.D.; Hj Idrus, R.B.; Abdul Rahman, M.R.; Sulaiman, N. Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review. Front. Pharmacol. 2021, 12, 3266. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Porciello, G.; Montagnese, C.; Crispo, A.; Grimaldi, M.; Libra, M.; Vitale, S.; Palumbo, E.; Pica, R.; Calabrese, I.; Cubisino, S.; et al. Mediterranean diet and quality of life in women treated for breast cancer: A baseline analysis of DEDiCa multicentre trial. PLoS ONE 2020, 15, e0239803. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Fernández del Río, L.; Gutiérrez-Casado, E.; Varela-López, A.; Villalba, J.M. Olive Oil and the Hallmarks of Aging. Molecules 2016, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Kamil, K.; Yazid, M.D.; Idrus, R.B.; Kumar, J. Hydroxytyrosol Promotes Proliferation of Human Schwann Cells: An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4404. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Medori, M.C.; Caruso, P.; Manganotti, P.; Chiurazzi, P.; Bertelli, M. Dietary supplements in neurological diseases and brain aging. J. Prev. Med. Hyg. 2022, 63, E174–E188. [Google Scholar] [CrossRef]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Canudas, S.; Becerra-Tomás, N.; Hernández-Alonso, P.; Galié, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, Y.; Meinilä, J.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Besnard, G.; Khadari, B.; Baradat, P.; Bervillé, A. Olea europaea (Oleaceae) phylogeography based on chloroplast DNA polymorphism. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 2002, 104, 1353–1361. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Canyelles, M.; Fitó, M.; Escolà-Gil, J.C. Effects of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on Lipoprotein Atherogenicity. Nutrients 2020, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Riley, F.R. Olive oil production on bronze age crete: Nutritional properties, processing methods and storage life of Minoan olive oil. Oxford J. Archaeol. 2002, 21, 63–75. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef]
- Aparicio, R.; Harwood, J. Handbook of Olive Oil: Analysis and Properties; Springer: New York, NY, USA, 2013. [Google Scholar]
- Vidal, A.M.; Moya, M.; Alcalá, S.; Romero, I.; Espínola, F. Enrichment of Refined Olive Oils with Phenolic Extracts of Olive Leaf and Exhausted Olive Pomace. Antioxidants 2022, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Machado, N.; Sobreira, C.; Domínguez-Perles, R.; Gomes, S.; Rosa, E.; Barros, A. Critical Review on the Significance of Olive Phytochemicals in Plant Physiology and Human Health. Molecules 2017, 22, 1986. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug. Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek Virgin Olive Oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Zanoni, B.; Breschi, C.; Mulinacci, N. An effective HPLC-based approach for the evaluation of the content of total phenolic compounds transferred from olives to virgin olive oil during the olive milling process. J. Sci. Food Agric. 2018, 98, 3636–3643. [Google Scholar] [CrossRef]
- Gariboldi, P.; Jommi, G.; Verotta, L. Secoiridoids from Olea europaea. Phytochemistry 1986, 25, 865–869. [Google Scholar] [CrossRef]
- Bastoni, L.; Bianco, A.; Piccioni, F.; Uccella, N. Biophenolic profile in olives by nuclear magnetic resonance. Food Chem. 2001, 73, 145–151. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, J.Y.; Park, C.S.; Shetty, K.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef]
- Goldsmith, C.; Stathopoulos, C.; Golding, J.; Roach, P. Fate of the phenolic compounds during olive oil production with the traditional press method. Int. Food Res. J. 2014, 21, 101–109. [Google Scholar]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole Lyophilized Olives as Sources of Unexpectedly High Amounts of Secoiridoids: The Case of Three Tuscan Cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in Table Olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. Efsa J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharm. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Auñon-Calles, D.; Giordano, E.; Bohnenberger, S.; Visioli, F. Hydroxytyrosol is not genotoxic in vitro. Pharm. Res. 2013, 74, 87–93. [Google Scholar] [CrossRef]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free. Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef]
- Kutlu, T.; Özkan, H.; Güvenç, M. Tyrosol retards induction of fibrosis in rats. J. Food Biochem. 2021, 45, e13965. [Google Scholar] [CrossRef]
- Li, X.; Wei, T.; Li, J.; Yuan, Y.; Wu, M.; Chen, F.; Deng, Z.Y.; Luo, T. Tyrosol Ameliorates the Symptoms of Obesity, Promotes Adipose Thermogenesis, and Modulates the Composition of Gut Microbiota in HFD Fed Mice. Mol. Nutr. Food Res. 2022, 66, e2101015. [Google Scholar] [CrossRef]
- Chernysheva, G.A.; Smol’yakova, V.I.; Osipenko, A.N.; Plotnikova, T.M.; Plotnikov, M.B. Effect of p-Tyrosol on the Process of Left-Ventricular Remodeling in the Long Period after Myocardial Infarction. Bull. Exp. Biol. Med. 2022, 173, 17–20. [Google Scholar] [CrossRef]
- Dávalos, J.Z.; Valderrama-Negrón, A.C.; Barrios, J.R.; Freitas, V.L.S.; Ribeiro da Silva, M. Energetic and Structural Properties of Two Phenolic Antioxidants: Tyrosol and Hydroxytyrosol. J. Phys. Chem. A 2018, 122, 4130–4137. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; Reyes, J.J.; Benitez-Guerrero, A.; Espartero, J.L.; González-Correa, J.A. Differences in the Neuroprotective Effect of Orally Administered Virgin Olive Oil (Olea europaea) Polyphenols Tyrosol and Hydroxytyrosol in Rats. J. Agric. Food Chem. 2015, 63, 5957–5963. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, Y.-J.; Kim, M.J.; Ahn, J.; Ha, T.-Y.; Lee, S.H.; Jang, Y.J.; Jung, C.H. Pharmacokinetics of Tyrosol Metabolites in Rats. Molecules 2016, 21, 128. [Google Scholar] [CrossRef]
- Kritek, P.A.; Levy, B.D. Approach to the Patient with Disease of the Respiratory System. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Visca, D.; Ong, C.; Tiberi, S.; Centis, R.; D’ambrosio, L.; Chen, B.; Mueller, J.; Mueller, P.; Duarte, R.; Dalcolmo, M. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.C.; Sonnappa, S. Pneumonia and other respiratory infections. Pediatr. Clin. N. Am. 2009, 56, 135–156. [Google Scholar] [CrossRef]
- D’amato, G.; Vitale, C.; De Martino, A.; Viegi, G.; Lanza, M.; Molino, A.; Sanduzzi, A.; Vatrella, A.; Annesi-Maesano, I.; D’amato, M. Effects on asthma and respiratory allergy of Climate change and air pollution. Multidiscip. Respir. Med. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Le Souëf, P.N.; Candelaria, P.; Goldblatt, J. Evolution and respiratory genetics. Eur. Respir. J. 2006, 28, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.G.; Balter, M.S.; Bell, A.D.; Kim, H.; McIvor, R.A. Diagnosis of asthma in adults. CMAJ 2009, 181, E210–E220. [Google Scholar] [CrossRef]
- Lutfiyya, M.N.; Henley, E.; Chang, L.F.; Reyburn, S.W. Diagnosis and treatment of community-acquired pneumonia. Am. Fam. Physician 2006, 73, 442–450. [Google Scholar]
- Byrne, A.L.; Marais, B.J.; Mitnick, C.D.; Lecca, L.; Marks, G.B. Tuberculosis and chronic respiratory disease: A systematic review. Int. J. Infect. Dis. 2015, 32, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Small, P.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2011, 7, S3. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Meltzer, E.O.; Seiner, J.C.; Storms, W. Prevalence of allergic rhinitis in the United States. J. Allergy Clin. Immunol. 1997, 99, S808–S814. [Google Scholar] [CrossRef]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef]
- Berry, C.E.; Wise, R.A. Mortality in COPD: Causes, Risk Factors, and Prevention. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 375–382. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch Iii, J.P.; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef]
- Macneal, K.; Schwartz, D.A. The genetic and environmental causes of pulmonary fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 120–125. [Google Scholar] [CrossRef]
- Walter, N.; Collard, H.R.; King, T.E., Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.; Ren, C.L. Review of cystic fibrosis. Pediatr. Ann. 2019, 48, e154–e161. [Google Scholar] [CrossRef]
- Naehrig, S.; Chao, C.-M.; Naehrlich, L. Cystic fibrosis: Diagnosis and treatment. Dtsch. Ärzteblatt Int. 2017, 114, 564. [Google Scholar]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef]
- Falk, S.; Williams, C. Lung Cancer; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Yang, J.; Lin, J.; Liu, T.; Chen, T.; Pan, S.; Huang, W.; Li, S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer 2014, 85, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bunn, P.A., Jr.; Carney, D.N. Overview of chemotherapy for small cell lung cancer. Semin. Oncol. 1997, 24, S7–S69. [Google Scholar] [PubMed]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Bello, S.; Mincholé, E.; Fandos, S.; Lasierra, A.B.; Ruiz, M.A.; Simon, A.L.; Panadero, C.; Lapresta, C.; Menendez, R.; Torres, A. Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm. Med. 2014, 14, 123. [Google Scholar] [CrossRef]
- Arora, V.K.; Chopra, K.K. Inflammation plays a central role in respiratory diseases, including tuberculosis. Indian. J. Tuberc. 2018, 65, 103–105. [Google Scholar] [CrossRef]
- Spinelli Oliveira, E.; Hancock, J.T.; Hermes-Lima, M.; Isola, D.A.; Ochs, M.; Yu, J.; Wilhem Filho, D. Implications of dealing with airborne substances and reactive oxygen species: What mammalian lungs, animals, and plants have to say? Integr. Comp. Biol. 2007, 47, 578–591. [Google Scholar] [CrossRef]
- Kay, E.; Scotland, R.S.; Whiteford, J.R. Toll-like receptors: Role in inflammation and therapeutic potential. Biofactors 2014, 40, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Adler, K.B.; Fischer, B.M.; Wright, D.T.; Cohn, L.A.; Becker, S. Interactions between respiratory epithelial cells and cytokines: Relationships to lung inflammation. Ann. N. Y. Acad. Sci. 1994, 725, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Steinfort, D.P.; Smallwood, D.; Hew, M.; Chen, W.; Ernst, M.; Irving, L.B.; Anderson, G.P.; Hibbs, M.L. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs. Mucosal Immunol. 2016, 9, 550–563. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Shuai, W.; Meng, J.; Feng, J.; Han, Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020, 69, 883–895. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Sun, J.; Wang, T.; Liu, Q.; Wu, G.; Qian, Y.; Yang, W.; Wang, Y.; Wang, W. Cigarette smoke promotes oral leukoplakia via regulating glutamine metabolism and M2 polarization of macrophage. Int. J. Oral. Sci. 2021, 13, 25. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Levy, H.; Kalish, L.A.; Huntington, I.; Weller, N.; Gerard, C.; Silverman, E.K.; Celedón, J.C.; Pier, G.B.; Weiss, S.T. Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 256–262. [Google Scholar] [CrossRef]
- Heidari, B. The importance of C-reactive protein and other inflammatory markers in patients with chronic obstructive pulmonary disease. Casp. J. Intern. Med. 2012, 3, 428–435. [Google Scholar]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Fois, S.S.; Piras, B.; Carru, C.; Pirina, P. Reliability and Usefulness of Different Biomarkers of Oxidative Stress in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2020, 2020, 4982324. [Google Scholar] [CrossRef] [PubMed]
- Fezai, M.; Senovilla, L.; Jemaà, M.; Ben-Attia, M. Analgesic, Anti-Inflammatory and Anticancer Activities of Extra Virgin Olive Oil. J. Lipids 2013, 2013, 129736. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chow, M.P.; Huang, W.C.; Lin, Y.C.; Chang, Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: Structure-activity relationships. Mol. Pharm. 2004, 66, 683–693. [Google Scholar]
- Park, N.; Park, S.J.; Kim, M.H.; Yang, W.M. Efficacy and mechanism of essential oil from Abies holophylla leaf on airway inflammation in asthma: Network pharmacology and in vivo study. Phytomedicine 2022, 96, 153898. [Google Scholar] [CrossRef]
- Dahl, M.; Vestbo, J.; Lange, P.; Bojesen, S.E.; Tybjærg-Hansen, A.; Nordestgaard, B.G. C-reactive Protein As a Predictor of Prognosis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, M.; Moradi Moghdam, O.; Niakan Lahiji, M.; Vahdat Shariatpanahi, Z. High-fat low-carbohydrate enteral feeding enriched with olive oil and acute respiratory failure: A double-blind, randomized, controlled trial. Clin. Nutr. ESPEN 2022, 52, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.U.; Mahmood, M.H.; Sama, N.U.; Afzal, M.; Asaruddin, M.R.; Khan, M.S.A. Impact of Olive Oil Constituents on C-reactive Protein: In silico Evidence. J. Oleo Sci. 2022, 71, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Cron, R.Q. COVID-19 cytokine storm: Targeting the appropriate cytokine. Lancet Rheumatol. 2021, 3, e236–e237. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Albiges, L.; Chaput, N.; Saada, V.; Pommeret, F.; Griscelli, F.; Balleyguier, C.; Besse, B.; Marabelle, A.; Netzer, F.; et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: A case report. Ann. Oncol. 2020, 31, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xi, R.; Zhang, Z.; Li, W.; Liu, Y.; Jin, F.; Wang, X. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1α in seawater aspiration-induced lung injury in rats. Int. J. Mol. Sci. 2014, 15, 12861–12884. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal. Transduct. Target. Ther. 2021, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Rungsung, S.; Singh, T.U.; Rabha, D.J.; Kumar, T.; Cholenahalli Lingaraju, M.; Parida, S.; Paul, A.; Sahoo, M.; Kumar, D. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine 2018, 110, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Yin, Y.; Zhang, Y.; Cao, Y.; Lin, X.; Huang, L.; Hoffmann, D.; Lu, M.; Qiu, Y. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11, 2063. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin. Nutr. 2011, 30, 533–540. [Google Scholar] [CrossRef]
- Ghorbel, I.; Chaâbane, M.; Boudawara, O.; Kamoun, N.G.; Boudawara, T.; Zeghal, N. Dietary unsaponifiable fraction of extra virgin olive oil supplementation attenuates lung injury and DNA damage of rats co-exposed to aluminum and acrylamide. Env. Sci. Pollut. Res. Int. 2016, 23, 19397–19408. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, S.; Kim, M.J.; Kang, B.C.; Dhakal, H.; Choi, Y.A.; Park, P.H.; Choi, H.; Shin, T.Y.; Choi, H.G.; et al. Tyrosol attenuates lipopolysaccharide-induced acute lung injury by inhibiting the inflammatory response and maintaining the alveolar capillary barrier. Food Chem. Toxicol. 2017, 109, 526–533. [Google Scholar] [CrossRef]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef]
- FitzGerald, G.A. Misguided drug advice for COVID-19. Science 2020, 367, 1434. [Google Scholar] [CrossRef] [PubMed]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, J.; Park, E.K.; Bae, J.S. Maslinic Acid Ameliorates Inflammation via the Downregulation of NF-kappaB and STAT-1. Antioxidants 2020, 9, 106. [Google Scholar] [CrossRef]
- Thangavel, N.; Al Bratty, M.; Al Hazmi, H.A.; Najmi, A.; Ali Alaqi, R.O. Molecular Docking and Molecular Dynamics Aided Virtual Search of OliveNet™ Directory for Secoiridoids to Combat SARS-CoV-2 Infection and Associated Hyperinflammatory Responses. Front. Mol. Biosci. 2020, 7, 627767. [Google Scholar] [CrossRef]
- Majumder, D.; Debnath, M.; Sharma, K.N.; Shekhawat, S.S.; Prasad, G.; Maiti, D.; Ramakrishna, S. Olive Oil Consumption can Prevent Non-communicable Diseases and COVID-19: A Review. Curr Pharm Biotechnol 2022, 23, 261–275. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Derouiche, S. Oxidative stress associated with SARS-CoV-2 (COVID-19) increases the severity of the lung disease-a systematic review. J. Infect. Dis. Epidemiol. 2020, 6, 121. [Google Scholar]
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of inflammatory cells in airway remodeling in COPD. Int. J. Chron. Obs. Pulmon Dis. 2018, 13, 3341–3348. [Google Scholar] [CrossRef]
- Lammi, C.; Arnoldi, A. Food-derived antioxidants and COVID-19. J. Food Biochem. 2021, 45, e13557. [Google Scholar] [CrossRef]
- Wallet, F.; Delannoy, B.; Haquin, A.; Debord, S.; Leray, V.; Bourdin, G.; Bayle, F.; Richard, J.-C.; Boussel, L.; Guérin, C. Evaluation of Recruited Lung Volume at Inspiratory Plateau Pressure With PEEP Using Bedside Digital Chest X-ray in Patients With Acute Lung Injury/ARDS. Respir. Care 2013, 58, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N. The role of intrapulmonary nitric oxide generation in the development of adult respiratory distress syndrome. Surg. Today 1999, 29, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Shao, X.; Liu, F.; Zhu, B.; Dong, Z.; Xu, W.; Yang, Q. Correlation between nitric oxide content in exhaled breath condensate and the severity of acute respiratory distress syndrome. Int. J. Clin. Exp. Pathol. 2017, 10, 7350–7355. [Google Scholar] [PubMed]
- Guimarães, L.M.F.; Rossini, C.V.T.; Lameu, C. Implications of SARS-Cov-2 infection on eNOS and iNOS activity: Consequences for the respiratory and vascular systems. Nitric Oxide 2021, 111–112, 64–71. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Xia, Y.M.; Yang, B.; Su, X.N.; Chen, J.K.; Li, W.; Jiang, T. Protective Effects of Tyrosol against LPS-Induced Acute Lung Injury via Inhibiting NF-κB and AP-1 Activation and Activating the HO-1/Nrf2 Pathways. Biol. Pharm. Bull. 2017, 40, 583–593. [Google Scholar] [CrossRef]
- Majumder, D.; Debnath, R.; Nath, P.; Libin Kumar, K.V.; Debnath, M.; Tribedi, P.; Maiti, D. Bromelain and Olea europaea (L.) leaf extract mediated alleviation of benzo(a)pyrene induced lung cancer through Nrf2 and NFκB pathway. Env. Sci. Pollut. Res. Int. 2021, 28, 47306–47326. [Google Scholar] [CrossRef]
- Alaçam, H.; Karli, R.; Alici, O.; Avci, B.; Güzel, A.; Kozan, A.; Mertoglu, C.; Murat, N.; Salis, O.; Güzel, A.; et al. The effects of α-tocopherol on oxidative damage and serum levels of Clara cell protein 16 in aspiration pneumonitis induced by bile acids. Hum. Exp. Toxicol. 2013, 32, 53–61. [Google Scholar] [CrossRef]
- Dikmen, N.; Cellat, M.; Etyemez, M.; İşler, C.T.; Uyar, A.; Aydın, T.; Güvenç, M. Ameliorative Effects of Oleuropein on Lipopolysaccharide-Induced Acute Lung Injury Model in Rats. Inflammation 2021, 44, 2246–2259. [Google Scholar] [CrossRef]
- Cellat, M.; Kuzu, M.; İşler, C.T.; Etyemez, M.; Dikmen, N.; Uyar, A.; Gökçek, İ.; Türk, E.; Güvenç, M. Tyrosol improves ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Naunyn Schmiedebergs Arch. Pharm. 2021, 394, 2061–2075. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Yang, J.; Xia, Y.; Tong, D.N.; Zaloga, G.P.; Qin, H.L. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: A prospective, randomized, multi-center clinical trial in China. Nutr. J. 2015, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Moore, R.; Braakhuis, A. The Effect of Olive Leaf Extract on Upper Respiratory Illness in High School Athletes: A Randomised Control Trial. Nutrients 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; De San Pedro, B.S.; Millán, C.; Panizo, C.; Martín, S.; Florido, F. Exploratory study of tolerability and immunological effect of a short up-dosing immunotherapy phase with a standardised allergen extract derived from pollen of Olea europaea. Clin. Transl. Allergy 2015, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Jaudszus, A.; Mainz, J.G.; Pittag, S.; Dornaus, S.; Dopfer, C.; Roth, A.; Jahreis, G. Effects of a dietary intervention with conjugated linoleic acid on immunological and metabolic parameters in children and adolescents with allergic asthma--a placebo-controlled pilot trial. Lipids Health Dis. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Oropharyngeal Immunoprophylaxis with High Polyphenolic Olive Oil as Clinical Spectrum Mitigating Factor in COVID-19. (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/record/NCT05685901?term=olive&cond=Respiratory+Disease&draw=2&rank=8 (accessed on 30 April 2023).
- ClinicalTrials.gov. Standardized Olive Leaf Capsules; As a Co-Therapy in the Treatment of COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04873349?term=olive&cond=Respiratory+Disease&draw=2&rank=9 (accessed on 30 April 2023).
- clinicalTrials.gov. Safety Clinical Trial with Depigopid 50% Grasses/50% Olea Europaea (2000DPP/mL) or Depigoid 50% Grasses/50% Parietaria Judaica (2000DPP/mL). Available online: https://clinicaltrials.gov/ct2/show/record/NCT01734265?term=olive&cond=Respiratory+Disease&draw=3&rank=15 (accessed on 30 April 2023).
- ClinicalTrials.gov. The Effect of Intravenous Lipids on Lung Function in Acute Respiratory Distress Syndrome (ARDS). Available online: https://clinicaltrials.gov/ct2/show/record/NCT01096771?term=olive&cond=Respiratory+Disease&draw=5&rank=33 (accessed on 30 April 2023).
- Euba, B.; López-López, N.; Rodríguez-Arce, I.; Fernández-Calvet, A.; Barberán, M.; Caturla, N.; Martí, S.; Díez-Martínez, R.; Garmendia, J. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by nontypeable Haemophilus influenzae. Sci. Rep. 2017, 7, 12860. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharm. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. 2007, 12, 331–342. [Google Scholar]
- Alkhatib, A. Antiviral Functional Foods and Exercise Lifestyle Prevention of Coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Troy, N.M.; Bosco, A. Respiratory viral infections and host responses; insights from genomics. Respir. Res. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Ben Amor, I.; Gargouri, B.; Attia, H.; Zaabi, R.; Chira, A.B.; Saoudi, M.; Piperno, A.; Trischitta, P.; Tamburello, M.P.; et al. Analysis of Antioxidant and Antiviral Effects of Olive (Olea europaea L.) Leaf Extracts and Pure Compound Using Cancer Cell Model. Biomolecules 2023, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M.; et al. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antivir. Res. 2009, 83, 35–44. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Antunes, B.S.; do Nascimento, G.O.; Kawall, J.C.S.; Oliveira, J.V.B.; Silva, K.; Costa, M.A.T.; Oliveira, C.R. Antiviral activity of medicinal plant-derived products against SARS-CoV-2. Exp. Biol. Med. 2022, 247, 1797–1809. [Google Scholar] [CrossRef]
- Geromichalou, E.G.; Geromichalos, G.D. In Silico Approach for the Evaluation of the Potential Antiviral Activity of Extra Virgin Olive Oil (EVOO) Bioactive Constituents Oleuropein and Oleocanthal on Spike Therapeutic Drug Target of SARS-CoV-2. Molecules 2022, 27, 7572. [Google Scholar] [CrossRef]
- Schultz, M.A.; Hagan, S.S.; Datta, A.; Zhang, Y.; Freeman, M.L.; Sikka, S.C.; Abdel-Mageed, A.B.; Mondal, D. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS ONE 2014, 9, e87204. [Google Scholar] [CrossRef]
- Hassan, S.M.; Jawad, M.J.; Ahjel, S.W.; Singh, R.B.; Singh, J.; Awad, S.M.; Hadi, N.R. The Nrf2 Activator (DMF) and COVID-19: Is there a Possible Role? Med. Arch. 2020, 74, 134–138. [Google Scholar] [CrossRef]
- Demerlé, C.; Gorvel, L.; Olive, D. BTLA-HVEM Couple in Health and Diseases: Insights for Immunotherapy in Lung Cancer. Front. Oncol. 2021, 11, 682007. [Google Scholar] [CrossRef]
- Torić, J.; Marković, A.K.; Brala, C.J.; Barbarić, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Uzu, E.; Yoshigai, Y.; Kato, C.; Tagami, M. Oleic acid and oleoylethanolamide decrease interferon-γ-induced expression of PD-L1 and induce apoptosis in human lung carcinoma cells. Eur. J. Pharm. 2021, 903, 174116. [Google Scholar] [CrossRef] [PubMed]
- Gallazzi, M.; Festa, M.; Corradino, P.; Sansone, C.; Albini, A.; Noonan, D.M. An Extract of Olive Mill Wastewater Downregulates Growth, Adhesion and Invasion Pathways in Lung Cancer Cells: Involvement of CXCR4. Nutrients 2020, 12, 903. [Google Scholar] [CrossRef]
- Siddique, A.B.; Kilgore, P.C.S.R.; Tajmim, A.; Singh, S.S.; Meyer, S.A.; Jois, S.D.; Cvek, U.; Trutschl, M.; Sayed, K.A.E. (−)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 2020, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, G.; Giaccone, G. C-MET inhibitors for advanced non-small cell lung cancer. Expert. Opin. Investig. Drugs 2018, 27, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Che, J.; Jänne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef]
- Razali, R.A.; Yazid, M.D.; Saim, A.; Idrus, R.B.H.; Lokanathan, Y. Approaches in Hydroxytyrosol Supplementation on Epithelial—Mesenchymal Transition in TGFβ1-Induced Human Respiratory Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 3974. [Google Scholar]




| Saponifiable Fraction | Unsaponifiable Fraction |
|---|---|
|
|
| Phenolic Acids | Phenolic Alcohols | Flavonoids | Secoroidoids | Lignans | Tocopherols and Tocotrienols |
|---|---|---|---|---|---|
|
|
|
|
| (α, β, γ, δ) |
| Category | Examples |
|---|---|
| Infections Disease caused by various microorganisms, such as bacteria, viruses, fungi and parasites. | Sinusitis, pneumonia, tuberculosis, influenza and COVID-19 |
| Obstructive Disease caused by narrowing or obstruction of the airways, causing difficulty in breathing. | Asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis and bronchiolitis |
| Restrictive Disease affects the lung tissue or chest wall making it difficult for the lungs to expand and contract properly. | COPD, pulmonary fibrosis and chest wall disorders |
| Occupational Disease caused by exposure to various substances in the workplace, such as dust, chemicals and fumes. | Occupational asthma and pneumoconiosis |
| Genetic Disease caused by genetic mutations or abnormalities. | Cystic fibrosis and alpha-1 antitrypsin deficiency |
| Malignancy Disease caused by the uncontrolled growth of abnormal cells in the lungs or airways. | Lung cancer and mesothelioma |
| Vascular Disease affects the blood vessels in the lungs. | Pulmonary hypertension and pulmonary embolism |
| Compound | Model | Dosage and Duration | Finding(s) | Possible Contribution in Respiratory Disease | Reference |
|---|---|---|---|---|---|
| 4-Hydroxyphenylacetic acid (4-HPA) |
|
|
| Anti-inflammation | [118] |
| Luteolin | Caecal ligation and puncture (CLP)-induced ALI mice | 0.2 mg/kg 1 h |
|
| [120] |
| Oleuropein aglycone | Carrageenan-induced pleurisy mice | 40–100 mM/kg 30 min |
| Anti-inflammation | [122] |
| Hydrophilic fraction from olive oil (OOHF) | Aluminium- and acrylamide-induced rats | (1 mL) by gavage—21 days |
| Antioxidant | [123] |
| Tyrosol |
|
|
| Anti-inflammation | [124] |
| Maslinic acid |
|
|
|
| [129] |
| Pre-senescent human lung cells (MRC5) |
|
| Anti-inflammation | [128] |
| Ethanolic olive leaf extract (EOLE) and bromelain | Benzo(a)pyrene-mediated lung cancer mice |
|
|
| [142] |
| α-tocopherol (AT) | Aspiration-pneumonitis-induced rats |
|
| Antioxidant | [143] |
| Oleuropein | Lipopolysaccharide (LPS)-induced ALI in rats |
|
|
| [144] |
| Tyrosol | Ovalbumin-induced asthma rats |
|
|
| [145] |
| ID | Intervention and Control | Disease/ Condition | Study Size | Finding | References |
|---|---|---|---|---|---|
| NCT02421614 |
| Acute respiratory failure | 48 ventilated acute pulmonary failure patients |
| [111] |
| NCT01674595 |
| Allergic rhinoconjunctivitis | 93 allergic rhinoconjunctivitis with/without asthma |
| [148] |
| NCT00876356 |
| Asthma | 29 asthmatic children |
| [149] |
| ACTRN12618000328279 |
| Upper respiratory illness | 32 high school athletes |
| [147] |
| N/A | 226 patients undergoing surgery |
| [146] |
| ClinicalTrials.gov ID | Intervention and Control | Disease/Condition | Study Size | Findings/Outcome Measures | Status | References |
|---|---|---|---|---|---|---|
| NCT05685901 |
| COVID-19 prevention | 88 adults |
|
| [150] |
| NCT04873349 |
|
| 60 COVID-19 adults |
|
| [151] |
| NCT01734265 |
|
| 63 adults |
|
| [152] |
| NCT01096771 |
| Acute respiratory distress syndrome | 14 |
|
| [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijakumaran, U.; Goh, N.-Y.; Razali, R.A.; Abdullah, N.A.H.; Yazid, M.D.; Sulaiman, N. Role of Olive Bioactive Compounds in Respiratory Diseases. Antioxidants 2023, 12, 1140. https://doi.org/10.3390/antiox12061140
Vijakumaran U, Goh N-Y, Razali RA, Abdullah NAH, Yazid MD, Sulaiman N. Role of Olive Bioactive Compounds in Respiratory Diseases. Antioxidants. 2023; 12(6):1140. https://doi.org/10.3390/antiox12061140
Chicago/Turabian StyleVijakumaran, Ubashini, Neng-Yao Goh, Rabiatul Adawiyah Razali, Nur Atiqah Haizum Abdullah, Muhammad Dain Yazid, and Nadiah Sulaiman. 2023. "Role of Olive Bioactive Compounds in Respiratory Diseases" Antioxidants 12, no. 6: 1140. https://doi.org/10.3390/antiox12061140
APA StyleVijakumaran, U., Goh, N. -Y., Razali, R. A., Abdullah, N. A. H., Yazid, M. D., & Sulaiman, N. (2023). Role of Olive Bioactive Compounds in Respiratory Diseases. Antioxidants, 12(6), 1140. https://doi.org/10.3390/antiox12061140