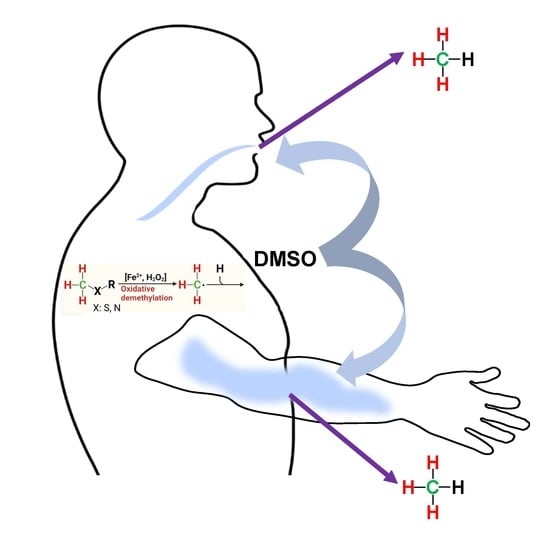
Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine
Abstract
:1. Introduction
1.1. Traditional View of Microbial Methane Formation in Humans
1.2. Alternative Mechanism(s) of Non-Microbial Methane Formation in Eukaryotes
1.3. Application of DMSO to Humans
1.4. Aims and Postulates
2. Materials and Methods
2.1. Subject, Materials, Experiments, and Sampling of Air
2.1.1. Subject of the Study
2.1.2. Materials: Position-Specific Isotopically Labeled DMSO and Methionine
2.1.3. Experiments and Sampling of Air
2.1.4. Oral Intake of 13C- and 2H-Labeled DMSO
2.1.5. Arm Incubations and Exposure to Solar Light
2.1.6. Blood Samples and Incubation with DMSO and Methionine
2.2. Analytical Measurements
2.2.1. Natural Abundance of 13C/12C and 2H/1H, Definition of δ Values, Isotopic Excess, and Keeling Method
2.2.2. Laser Absorption Spectroscopy—Cavity Ringdown Spectroscopy
Measurements of CH4 Concentrations and Stable Carbon Isotope Values
2.2.3. Measurements of CH4 Concentrations Using Gas Chromatography Flame Ionization Detection
2.2.4. Continuous Flow Isotope Ratio Mass Spectrometry
Measurement of δ13C-CH4 Values
Measurement of δ2H-CH4 Values
2.3. Statistics
3. Results
3.1. Oral Intake of Isotopically Labeled DMSO and Measurements of Breath Air
3.2. Blood Samples and Addition of Isotopically Labeled DMSO and Methionine
3.3. Skin Application of Isotopically Labeled DMSO and Incubation of Arm with Exposure to Natural Sunlight
4. Discussion
4.1. Conversion of Methylated Sulfur Compounds to Methane
4.2. Oral Administration of 13C-Labeled DMSO
4.3. Supplementation of 13C-Labeled DMSO and Methionine to Blood Samples
4.4. Dermal CH4 Emissions after Treatment of Isotopically Labeled DMSO
4.5. ROS-Induced non-Microbial Formation of CH4 from Methylated S-/N-Compounds in Humans: A Hypothesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AscariteII® | sodium-hydroxide-coated silica |
| C | carbon |
| CH4 | methane |
| •CH3 | methyl radicals |
| CO3–• | carbonate radicals |
| CO2 | carbon dioxide |
| CRDS | cavity ringdown spectroscopy |
| D | deuterium |
| DMSO | dimethyl sulfoxide |
| Drierite® | anhydrous calcium sulfate |
| ER | endoplasmatic reticulum |
| EDTA | Ethylenediaminetetraacetic acid |
| FDA | Food and Drug Administration |
| [FeIV=O]2+ | nonheme oxo-iron(IV) |
| Fe2+ | ferrous iron |
| Fe3+ | ferric iron |
| FID | flame ionization detector |
| GC | gas chromatography |
| GPC | L-alpha-glycerylphosphorylcholine |
| H2 | hydrogen |
| H2O2 | hydrogen peroxide |
| IAEA | International Atomic Energy Agency |
| IRMS | isotopic ratio mass spectrometry |
| N | nitrogen |
| NIST | National Institute of Standards and Technology |
| •OH | hydroxyl radicals |
| O2–• | superoxide radicals |
| PI | principal investigator |
| ppvb | parts per billion by volume |
| ppmv | parts per million by volume |
| PTFE | polytetrafluorethylene |
| RET | reverse electron transport |
| ROS | reactive oxygen species |
| S | sulfur |
| SAM | S-adenosyl methionine |
| SD | standard deviation |
| SI | system of units |
| TC | thermal conversion |
| mUr | milliurey |
| V-PDB | Vienna Pee Dee Belemnite |
| V-SMOW | Vienna Standard Mean Ocean Water |
References
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.G.; Brass, M.; Rockmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Zhu, Q.; Shen, Y.; Wang, X.; Wang, M.; Peng, C. A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: An overview. Atmos. Environ. 2015, 115, 26–35. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Unravelling the effects of blue light on aerobic methane emissions from canola. J. Plant Physiol. 2019, 233, 12–19. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B.W. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008, 180, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaderi, M.M.; Reid, D.M. Methane emissions from six crop species exposed to three components of global climate change: Temperature, ultraviolet-B radiation and water stress. Physiol. Plant. 2009, 137, 139–147. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Chang, S.X.; Chen, H.; Han, X.-G. Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Sci. Rev. 2013, 127, 193–202. [Google Scholar] [CrossRef]
- Lenhart, K.; Althoff, F.; Greule, M.; Keppler, F. Technical Note: Methionine, a precursor of methane in living plants. Biogeosciences 2015, 12, 1907–1914. [Google Scholar] [CrossRef] [Green Version]
- Tuboly, E.; Szabo, A.; Garab, D.; Bartha, G.; Janovszky, A.; Eros, G.; Szabo, A.; Mohacsi, A.; Szabo, G.; Kaszaki, J.; et al. Methane biogenesis during sodium azide-induced chemical hypoxia in rats. Am. J. Physiol.-Cell Physiol. 2013, 304, C207–C214. [Google Scholar] [CrossRef]
- Ghyczy, M.; Torday, C.; Kaszaki, J.; Szabo, A.; Czobel, M.; Boros, M. Hypoxia-induced generation of methane in mitochondria and eukaryotic cells—An alternative approach to methanogenesis. Cell. Physiol. Biochem. 2008, 21, 251–258. [Google Scholar] [CrossRef]
- Lenhart, K.; Bunge, M.; Ratering, S.; Neu, T.R.; Schüttmann, I.; Greule, M.; Kammann, C.; Schnell, S.; Müller, C.; Zorn, H.; et al. Evidence for methane production by saprotrophic fungi. Nat. Commun. 2012, 3, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroll, M.; Keppler, F.; Greule, M.; Eckhardt, C.; Zorn, H.; Lenhart, K. The stable carbon isotope signature of methane produced by saprotrophic fungi. Biogeosciences 2020, 17, 3891–3901. [Google Scholar] [CrossRef]
- Hartmann, J.F.; Günthel, M.; Klintzsch, T.; Kirillin, G.; Grossart, H.-P.; Keppler, F.; Isenbeck-Schröter, M. High Spatiotemporal Dynamics of Methane Production and Emission in Oxic Surface Water. Environ. Sci. Technol. 2020, 54, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Klintzsch, T.; Langer, G.; Wieland, A.; Geisinger, H.; Lenhart, K.; Nehrke, G.; Keppler, F. Effects of Temperature and Light on Methane Production of Widespread Marine Phytoplankton. J. Geophys. Res. Biogeosciences 2020, 125, e2020JG005793. [Google Scholar] [CrossRef]
- Lenhart, K.; Klintzsch, T.; Langer, G.; Nehrke, G.; Bunge, M.; Schnell, S.; Keppler, F. Evidence for methane production by the marine algae Emiliania huxleyi. Biogeosciences 2016, 13, 3163–3174. [Google Scholar] [CrossRef] [Green Version]
- Bižić, M.; Klintzsch, T.; Ionescu, D.; Hindiyeh, M.Y.; Günthel, M.; Muro-Pastor, A.M.; Eckert, W.; Urich, T.; Keppler, F.; Grossart, H.-P. Aquatic and terrestrial cyanobacteria produce methane. Sci. Adv. 2020, 6, eaax5343. [Google Scholar] [CrossRef] [Green Version]
- Ernst, L.; Steinfeld, B.; Barayeu, U.; Klintzsch, T.; Kurth, M.; Grimm, D.; Dick, T.P.; Rebelein, J.G.; Bischofs, I.B.; Keppler, F. Methane formation driven by reactive oxygen species across all living organisms. Nature 2022, 603, 482–487. [Google Scholar] [CrossRef]
- Boros, M.; Keppler, F. Methane Production and Bioactivity—A Link to Oxido-Reductive Stress. Front. Physiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Bond, J.H., Jr.; Engel, R.R.; Levitt, M.D. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J. Exp. Med. 1971, 133, 572–588. [Google Scholar] [CrossRef]
- Levitt, M.D.; Furne, J.K.; Kuskowski, M.; Ruddy, J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin. Gastroenterol. Hepatol. 2006, 4, 123–129. [Google Scholar] [CrossRef]
- Peled, Y.; Weinberg, D.; Hallak, A.; Gilat, T. Factors affecting methane production in humans. Dig. Dis. Sci. 1987, 32, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Keppler, F.; Schiller, A.; Ehehalt, R.; Greule, M.; Hartmann, J.; Polag, D. Stable isotope and high precision concentration measurements confirm that all humans produce and exhale methane. J. Breath Res. 2016, 10, 016003. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001, 48, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Polag, D.; Leiß, O.; Keppler, F. Age dependent breath methane in the German population. Sci. Total Environ. 2014, 481, 582–587. [Google Scholar] [CrossRef]
- Mello, C.S.; Tahan, S.; Melli, L.C.; Rodrigues, M.S.; de Mello, R.M.; Scaletsky, I.C.; de Morais, M.B. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J. Gastroenterol. 2012, 18, 5932–5939. [Google Scholar] [CrossRef]
- Pitt, P.; de Bruijn, K.M.; Beeching, M.F.; Goldberg, E.; Blendis, L.M. Studies on breath methane: The effect of ethnic origins and lactulose. Gut 1980, 21, 951–954. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllou, K.; Chang, C.; Pimentel, M. Methanogens, methane and gastrointestinal motility. J. Neurogastroenterol. Motil. 2014, 20, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Szabó, A.; Ruzsanyi, V.; Unterkofler, K.; Mohácsi, Á.; Tuboly, E.; Boros, M.; Szabó, G.; Hinterhuber, H.; Amann, A. Exhaled methane concentration profiles during exercise on an ergometer. J. Breath Res. 2015, 9, 016009. [Google Scholar] [CrossRef] [Green Version]
- Conway de Macario, E.; Macario, A.J. Methanogenic archaea in health and disease: A novel paradigm of microbial pathogenesis. Int. J. Med. Microbiol. 2009, 299, 99–108. [Google Scholar] [CrossRef]
- Furnari, M.; Savarino, E.; Bruzzone, L.; Moscatelli, A.; Gemignani, L.; Giannini, E.G.; Zentilin, P.; Dulbecco, P.; Savarino, V. Reassessment of the role of methane production between irritable bowel syndrome and functional constipation. J. Gastrointest. Liver Dis. 2012, 21, 157–163. [Google Scholar]
- Hwang, L.; Low, K.; Khoshini, R.; Melmed, G.; Sahakian, A.; Makhani, M.; Pokkunuri, V.; Pimentel, M. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig. Dis. Sci. 2010, 55, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, D.; Basseri, R.; Makhani, M.; Chong, K.; Chang, C.; Pimentel, M. Methane on Breath Testing Is Associated with Constipation: A Systematic Review and Meta-analysis. Dig. Dis. Sci. 2011, 56, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Montes, R.G.; Saavedra, J.M.; Perman, J.A. Relationship between methane production and breath hydrogen excretion in lactose-malabsorbing individuals. Dig. Dis. Sci. 1993, 38, 445–448. [Google Scholar] [CrossRef]
- Roccarina, D.; Lauritano, E.C.; Gabrielli, M.; Franceschi, F.; Ojetti, V.; Gasbarrini, A. The Role of Methane in Intestinal Diseases. Am. J. Gastroenterol. 2010, 105, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Polag, D.; Keppler, F. Global methane emissions from the human body: Past, present and future. Atmos. Environ. 2019, 214, 116823. [Google Scholar] [CrossRef]
- de Lacy Costello, B.P.; Ledochowski, M.; Ratcliffe, N.M. The importance of methane breath testing: A review. J. Breath Res. 2013, 7, 024001. [Google Scholar] [CrossRef]
- Polag, D.; Keppler, F. Long-term monitoring of breath methane. Sci. Total Environ. 2018, 624, 69–77. [Google Scholar] [CrossRef]
- Polag, D.; Keppler, F. COVID19-vaccination affects breath methane dynamics. bioRxiv 2022. [Google Scholar] [CrossRef]
- Boros, M.; Keppler, F. Production and Signaling of Methane. Gasotransmitters 2018, 12, 192. [Google Scholar]
- Abdulmajeed, A.M.; Derby, S.R.; Strickland, S.K.; Qaderi, M.M. Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. J. Photochem. Photobiol. B Biol. 2017, 166, 193–201. [Google Scholar] [CrossRef]
- Abdulmajeed, A.M.; Qaderi, M.M. Intrashoot variation in aerobic methane emissions from pea plants exposed to multiple abiotic stresses. Acta Physiol. Plant. 2017, 39, 124. [Google Scholar] [CrossRef]
- Bruhn, D.; Mikkelsen, T.N.; Obro, J.; Willats, W.G.T.; Ambus, P. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol. 2009, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.T.; Blei, E.; Fry, S.C.; Newman, M.F.; Reay, D.S.; Smith, K.A.; McLeod, A.R. Emission of methane, carbon monoxide, carbon dioxide and short-chain hydrocarbons from vegetation foliage under ultraviolet irradiation. Plant Cell Environ. 2015, 38, 980–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigano, I.; Röckmann, T.; Holzinger, R.; van Dijk, A.; Keppler, F.; Greule, M.; Brand, W.A.; Geilmann, H.; van Weelden, H. The stable isotope signature of methane emitted from plant material under UV irradiation. Atmos. Environ. 2009, 43, 5637–5646. [Google Scholar] [CrossRef]
- Vigano, I.; van Weelden, H.; Holzinger, R.; Keppler, F.; McLeod, A.; Rockmann, T. Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences 2008, 5, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Messenger, D.J.; McLeod, A.R.; Fry, S.C. The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 2009, 32, 1–9. [Google Scholar] [CrossRef]
- Althoff, F.; Jugold, A.; Keppler, F. Methane formation by oxidation of ascorbic acid using iron minerals and hydrogen peroxide. Chemosphere 2010, 80, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, D.; Moller, I.M.; Mikkelsen, T.N.; Ambus, P. Terrestrial plant methane production and emission. Physiol. Plant. 2012, 144, 201–209. [Google Scholar] [CrossRef]
- Bruhn, D.; Mikkelsen, T.N.; Rolsted, M.M.M.; Egsgaard, H.; Ambus, P. Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol. 2014, 16, 512–516. [Google Scholar] [CrossRef]
- Keppler, F.; Boros, M.; Frankenberg, C.; Lelieveld, J.; McLeod, A.; Pirttila, A.M.; Rockmann, T.; Schnitzler, J.P. Methane formation in aerobic environments. Environ. Chem. 2009, 6, 459–465. [Google Scholar] [CrossRef]
- Althoff, F.; Benzing, K.; Comba, P.; McRoberts, C.; Boyd, D.R.; Greiner, S.; Keppler, F. Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat. Commun. 2014, 5, 4205. [Google Scholar] [CrossRef] [Green Version]
- Benzing, K.; Comba, P.; Martin, B.; Pokrandt, B.; Keppler, F. Nonheme Iron-Oxo-Catalyzed Methane Formation from Methyl Thioethers: Scope, Mechanism, and Relevance for Natural Systems. Chem. Eur. J. 2017, 23, 10465–10472. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.; Clemente da Silva, E.; Arbilla, G. Oxidation mechanism of dimethyl sulfoxide (DMSO) by OH radical in liquid phase. Phys. Chem. Chem. Phys. 2008, 10, 6867–6879. [Google Scholar] [CrossRef] [PubMed]
- Herscu-Kluska, R.; Masarwa, A.; Saphier, M.; Cohen, H.; Meyerstein, D. Mechanism of the Reaction of Radicals with Peroxides and Dimethyl Sulfoxide in Aqueous Solution. Chem. Eur. J. 2008, 14, 5880–5889. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Mizrahi, A.; Marks, V.; Meyerstein, D. Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radic. Biol. Med. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Yu, G.-H.; Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Prousek, J. Fenton chemistry in biology and medicine. Pure Appl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Kaplan, J.; Ward, D.M. The essential nature of iron usage and regulation. Curr. Biol. 2013, 23, R642–R646. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic Carbon–Sulfur Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.W.; Herschler, R. Pharmacology of DMSO. Cryobiology 1986, 23, 14–27. [Google Scholar] [CrossRef]
- Amemori, S.; Iwakiri, R.; Endo, H.; Ootani, A.; Ogata, S.; Noda, T.; Tsunada, S.; Sakata, H.; Matsunaga, H.; Mizuguchi, M.; et al. Oral dimethyl sulfoxide for systemic amyloid A amyloidosis complication in chronic inflammatory disease: A retrospective patient chart review. J. Gastroenterol. 2006, 41, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [Green Version]
- Hanley, B.P.; Bains, W.; Church, G. Review of Scientific Self-Experimentation: Ethics History, Regulation, Scenarios, and Views Among Ethics Committees and Prominent Scientists. Rejuvenation Res. 2019, 22, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Einzmann, T.; Schroll, M.; Kleint, J.F.; Greule, M.; Keppler, F. Application of concentration and 2-dimensional stable isotope measurements of methane to constrain sources and sinks in a seasonally stratified freshwater lake. Front. Environ. Sci. 2022, 10, 865862. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B. Stable isotope deltas: Tiny, yet robust signatures in nature. Isot. Environ. Health Stud. 2012, 48, 393–409. [Google Scholar] [CrossRef]
- Miralles-Robledillo, J.M.; Torregrosa-Crespo, J.; Martínez-Espinosa, R.M.; Pire, C. DMSO Reductase Family: Phylogenetics and Applications of Extremophiles. Int. J. Mol. Sci. 2019, 20, 3349. [Google Scholar] [CrossRef] [Green Version]
- Le, C.; Bae, M.; Kiamehr, S.; Balskus, E.P. Emerging Chemical Diversity and Potential Applications of Enzymes in the DMSO Reductase Superfamily. Annu. Rev. Biochem. 2022, 91, 475–504. [Google Scholar] [CrossRef]
- Anbar, M.; Neta, P. A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. Int. J. Appl. Radiat. Isot. 1967, 18, 493–523. [Google Scholar] [CrossRef]
- Eberhardt, M.K.; Colina, R. The reaction of OH radicals with dimethyl sulfoxide. A comparative study of Fenton’s reagent and the radiolysis of aqueous dimethyl sulfoxide solutions. J. Org. Chem. 1988, 53, 1071–1074. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.; Yoon, J. Kinetics and mechanisms of DMSO (dimethylsulfoxide) degradation by UV/H2O2 process. Water Res. 2004, 38, 2579–2588. [Google Scholar] [CrossRef]
- Lerner, A.; Kornweitz, H.; Zilbermann, I.; Yardeni, G.; Saphier, M.; Bar Ziv, R.; Meyerstein, D. Radicals in ‘biologically relevant’ concentrations behave differently: Uncovering new radical reactions following the reaction of hydroxyl radicals with DMSO. Free Radic. Biol. Med. 2021, 162, 555–560. [Google Scholar] [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J.A. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Stratton, K. Free radicals in human skin before and after exposure to light. Arch. Biochem. Biophys. 1968, 123, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared Radiation-Induced Matrix Metalloproteinase in Human Skin: Implications for Protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Bekö, G.; Zannoni, N.; Pugliese, G.; Carrito, M.; Cera, N.; Moura, C.; Wargocki, P.; Vasconcelos, P.; Nobre, P.; et al. Human metabolic emissions of carbon dioxide and methane and their implications for carbon emissions. Sci. Total Environ. 2022, 833, 155241. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Unterkofler, K.; Hinterhuber, H.; Amann, A. Emission rates of selected volatile organic compounds from skin of healthy volunteers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 959, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.J.A. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Zastrow, L.; Doucet, O.; Ferrero, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J. Free Radical Threshold Value: A New Universal Body Constant. Ski. Pharmacol. Physiol. 2015, 28, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Tuboly, E.; Molnár, R.; Tőkés, T.; Turányi, R.N.; Hartmann, P.; Mészáros, A.T.; Strifler, G.; Földesi, I.; Siska, A.; Szabó, A.; et al. Excessive alcohol consumption induces methane production in humans and rats. Sci. Rep. 2017, 7, 7329. [Google Scholar] [CrossRef] [Green Version]
- Pearson, T.W.; Dawson, H.J.; Lackey, H.B. Naturally occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J. Agric. Food Chem. 1981, 29, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Ghyczy, M.; Torday, C.; Boros, M. Simultaneous generation of methane, carbon dioxide, and carbon monoxide from choline and ascorbic acid—A defensive mechanism against reductive stress? FASEB J. 2003, 17, 1124–1126. [Google Scholar] [CrossRef]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef]
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A systems biology approach to iron metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225. [Google Scholar]
- Basak, T.; Kanwar, R. Iron imbalance in cancer: Intersection of deficiency and overload. Cancer Med. 2022, 11, 3837–3853. [Google Scholar] [CrossRef]
- Boros, M.; Ghyczy, M.; Érces, D.; Varga, G.; Tokés, T.; Kupai, K.; Torday, C.; Kaszaki, J. The anti-inflammatory effects of methane. Crit. Care Med. 2012, 40, 1269–1278. [Google Scholar] [CrossRef]
- Juhász, L.; Tallósy, S.P.; Nászai, A.; Varga, G.; Érces, D.; Boros, M. Bioactivity of Inhaled Methane and Interactions With Other Biological Gases. Front. Cell Dev. Biol. 2022, 9, 824749. [Google Scholar] [CrossRef] [PubMed]
- Jász, D.K.; Szilágyi, Á.L.; Tuboly, E.; Baráth, B.; Márton, A.R.; Varga, P.; Varga, G.; Érces, D.; Mohácsi, Á.; Szabó, A.; et al. Reduction in hypoxia-reoxygenation-induced myocardial mitochondrial damage with exogenous methane. J. Cell. Mol. Med. 2021, 25, 5113–5123. [Google Scholar] [CrossRef] [PubMed]
- Benke, K.; Jász, D.K.; Szilágyi, Á.L.; Baráth, B.; Tuboly, E.; Márton, A.R.; Varga, P.; Mohácsi, Á.; Szabó, A.; Széll, Z.; et al. Methane supplementation improves graft function in experimental heart transplantation. J. Heart Lung Transplant. 2021, 40, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Keppler, F.; Ernst, L.; Polag, D.; Zhang, J.; Boros, M. ROS-driven cellular methane formation: Potential implications for health sciences. Clin. Transl. Med. 2022, 12, e905. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, J. Methane might be made by all living organisms. Nature 2022, 603, 396–397. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keppler, F.; Boros, M.; Polag, D. Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine. Antioxidants 2023, 12, 1381. https://doi.org/10.3390/antiox12071381
Keppler F, Boros M, Polag D. Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine. Antioxidants. 2023; 12(7):1381. https://doi.org/10.3390/antiox12071381
Chicago/Turabian StyleKeppler, Frank, Mihály Boros, and Daniela Polag. 2023. "Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine" Antioxidants 12, no. 7: 1381. https://doi.org/10.3390/antiox12071381
APA StyleKeppler, F., Boros, M., & Polag, D. (2023). Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine. Antioxidants, 12(7), 1381. https://doi.org/10.3390/antiox12071381