Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality
Abstract
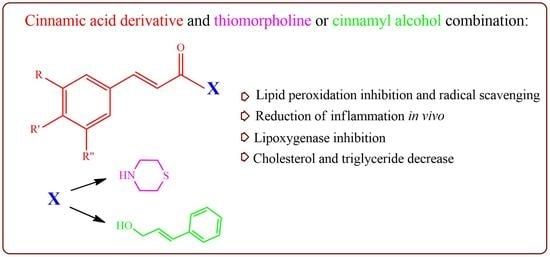
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
General Method for the Synthesis of the Compounds 1–7
2.3. In Vitro Biological Experiments
2.3.1. In Vitro Lipid Peroxidation
2.3.2. In Vitro Scavenging of the Stable Radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH)
2.3.3. In Vitro Evaluation of Lipoxygenase Activity
2.4. In Vivo Biological Experiments
2.4.1. Carrageenan-Induced Paw Edema
2.4.2. Effect on Plasma Cholesterol and Triglyceride Levels
3. Results and Discussion
3.1. Synthesis
3.2. Biological Evaluation
3.2.1. Effect on Lipid Peroxidation
3.2.2. Scavenging of DPPH
3.2.3. Effect on Acute Inflammation
3.2.4. Effect on Lipoxygenase
3.2.5. Effect of the Synthesized Compounds on Hyperlipidemia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolay, V.G.; Pavel, V.A.; Alexander, D.N.; Irina, L.Z.; Richard, O.J. Reactive oxygen species in pathogenesis of atherosclerosis. Curr. Pharm. Des. 2015, 21, 1134–1146. [Google Scholar]
- Moss, J.W.E.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.J. Lipid Metabolism in Inflammation and Immune Function. Nutrients 2022, 14, 1414. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxid. Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, G.F.; Csont, T.; Varga-Orvos, Z.; Puskás, L.G.; Murlasits, Z.; Ferdinandy, P. Expression of genes related to oxidative/nitrosative stress in mouse hearts: Effect of preconditioning and cholesterol diet. Med. Sci. Monit. 2010, 16, BR32–BR39. [Google Scholar]
- Varga, Z.V.; Kupai, K.; Szűcs, G.; Gáspár, R.; Pálóczi, J.; Faragó, N.; Zvara, A.; Puskás, L.G.; Rázga, Z.; Tiszlavicz, L.; et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell. Cardiol. 2013, 62, 111–121. [Google Scholar] [CrossRef]
- McCafferty, K.; Forbes, S.; Thiemermann, C.; Yaqoob, M.M. The challenge of translating ischemic conditioning from animal models to humans: The role of comorbidities. Dis. Model Mech. 2014, 7, 1321–1333. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch. Pharm. Res. 2007, 30, 64–74. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Kishina, M.; Koda, M.; Yamamoto, Y.; Tanaka, K.; Harada, Y.; Yoshida, A.; Hisatome, I. Nimesulide, a cyclooxygenase-2 selective inhibitor, suppresses obesity-related non-alcoholic fatty liver disease and hepatic insulin resistance through the regulation of peroxisome proliferator-activated receptor γ. Int. J. Mol. Med. 2016, 38, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef] [PubMed]
- De Lima, G.D.A.; Rodrigues, M.P.; de Mendes, T.A.O.; Moreira, G.A.; Siqueira, R.P.; da Silva, A.M.; Vaz, B.G.; Fietto, J.L.R.; Bressan, G.C.; Machado-Neves, M. Synthesis and Antimetastatic Activity Evaluation of Cinnamic Acid Derivatives Containing 1,2,3-Triazolic Portions. Toxicol. In Vitro 2018, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Pongsuwan, J.; Wungcharoen, C.; Yibchok-Anun, S. In Vitro Effects of Cinnamic Acid Derivatives on Protein Tyrosine Phosphatase 1B. J. Enzyme Inhib. Med. Chem. 2013, 28, 1067–1072. [Google Scholar] [CrossRef]
- Lan, J.S.; Hou, J.W.; Liu, Y.; Ding, Y.; Zhang, Y.; Li, L.; Zhang, T. Design, Synthesis and Evaluation of Novel Cinnamic Acid Derivatives Bearing N-Benzyl Pyridinium Moiety as Multifunctional Cholinesterase Inhibitors for Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2017, 32, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [Green Version]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Esters of some non-steroidal anti-inflammatory drugs with cinnamyl alcohol are potent lipoxygenase inhibitors with enhanced anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 5028–5031. [Google Scholar] [CrossRef]
- Asirvatham, S.; Thakor, E.; Jain, H. Morpholine and thiomorpholine: A privileged scaffold possessing diverse bioactivity profile. J. Chem. Rev. 2021, 3, 247–272. [Google Scholar]
- Tooulia, K.K.; Theodosis-Nobelos, P.; Rekka, E.A. Thiomorpholine derivatives with hypolipidemic and antioxidant activity. Arch. Pharm. 2015, 348, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cebeci, Y.U.; Bayrak, H.; Şirin, Y. Synthesis of novel Schiff bases and azol-β-lactam derivatives starting from morpholine and thiomorpholine and investigation of their antitubercular, antiurease activity, acethylcolinesterase inhibition effect and antioxidant capacity. Bioorg. Chem. 2019, 88, 102928. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Kourounakis, P.Ν.; Rekka, E.A. Anti-inflammatory and Hypolipidemic Effect of Novel Conjugates with Trolox and Other Antioxidant Acids. Med. Chem. 2017, 13, 214–225. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Kourounakis, P.N.; Rekka, E.A. Active Anti-Inflammatory and Hypolipidemic Derivatives of Lorazepam. EA. Molecules 2019, 24, 3277. [Google Scholar] [CrossRef] [Green Version]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Rekka, E.A. Nipecotic Acid Derivatives as Potent Agents against Neurodegeneration: A Preliminary Study. Molecules 2022, 27, 6984. [Google Scholar] [CrossRef]
- Reddy, P.R.; Reddy, G.M.; Padmaja, A.; Padmavathi, V.; Kondaiah, P.; Krishna, N.S. Synthesis, antioxidant, and cytotoxic activities of N-azole substituted thiomorpholine derivatives. Arch. Pharm. 2014, 347, 221–228. [Google Scholar] [CrossRef]
- Matralis, A.N.; Kourounakis, A.P. Optimizing the Pharmacological Profile of New Bifunctional Antihyperlipidemic/Antioxidant Morpholine Derivatives. ACS Med. Chem. Lett. 2018, 10, 98–104. [Google Scholar] [CrossRef]
- Miura, T.; Muraoka, S.; Fujimoto, Y. Lipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: Involvement of indomethacin radicals. Biochem. Pharmacol. 2002, 63, 2069–2074. [Google Scholar] [CrossRef]
- Afnan, S.A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [PubMed] [Green Version]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, M.; Ma, Q.Q.; Wu, Y.; Du, H.H.; Guo-Hua, G. Small molecule compounds with good anti-inflammatory activity reported in the literature from 01/2009 to 05/2021: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 2139–2159. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency. Molecules 2021, 26, 4060. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Politou, T.C.; Athanasekou, C.; Rekka, E.A. Improved Anti-inflammatory Activity and Potential Cytoprotective Properties of Tolfenamic Acid, Naproxen and Indomethacin Derivatives. Lett. Drug Des. Discov. 2017, 14, 464–475. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Ho, T.Y.; Lee, E.J.; Su, S.Y.; Tang, N.Y.; Hsieh, C.L. Ferulic Acid Reduces Cerebral Infarct Through Its Antioxidative and Anti-Inflammatory Effects Following Transient Focal Cerebral Ischemia in Rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J.L. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxid. Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef]
- Rezaei, S.; Hosseinimehr, S.J.; Zargari, M.; Karimpour, M.A.; Mirzaei, M.; Talebpour, A.F. Sinapic acid attenuates cyclophosphamide-induced liver toxicity in mice by modulating oxidative stress, NF-κB, and caspase-3. Iran J. Basic Med. Sci. 2023, 26, 526–531. [Google Scholar]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Shashkin, P.; Dragulev, B.; Ley, K. Macrophage differentiation to foam cells. Curr. Pharm. Des. 2005, 11, 3061–3072. [Google Scholar] [CrossRef] [Green Version]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Tziona, P.; Gavalas, A.; Kourounakis, P.N.; Rekka, E.A. Gabapentin Antioxidant Derivatives with Anti-Inflammatory and Neuro-protective Potency. Lett. Drug Des. Discov. 2022, 19, 579–590. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Pavić, K.; Perković, I.; Gilja, P.; Kozlina, F.; Ester, K.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Pontiki, E.; Zorc, B. Design, Synthesis and Biological Evaluation of Novel Primaquine-Cinnamic Acid Conjugates of the Amide and Acylsemicarbazide Type. Molecules 2016, 21, 1629. [Google Scholar] [CrossRef] [Green Version]
- Tuñón, J.; Badimón, L.; Bochaton-Piallat, M.L.; Cariou, B.; Daemen, M.J.; Egido, J.; Evans, P.C.; Hoefer, I.E.; Ketelhuth, D.F.J.; Lutgens, E.; et al. Identifying the anti-inflammatory response to lipid lowering therapy: A position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc. Res. 2019, 115, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Serban, C.; Sahebkar, A.; Mikhailidis, D.P.; Ursoniu, S.; Ray, K.K.; Rysz, J.; Toth, P.P.; Muntner, P.; Mosteoru, S.; et al. Impact of statin therapy on coronary plaque composition: A systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med. 2015, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Diamantis, E.; Kyriakos, G.; Quiles-Sanchez, L.V.; Farmaki, P.; Troupis, T. The anti-inflammatory effects of statins on coronary artery disease: An updated review of the literature. Curr. Cardiol. Rev. 2017, 13, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; de Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolenko, T.A.; Tuzikov, F.V.; Vasil’eva, E.D.; Cherkanova, M.S.; Tuzikova, N.A. Franctional composition of Blood Serum Lipoproteins in mice and rats with Triton WR 1339-Induced Lipidemia. Bull. Exp. Biol. Med. 2010, 149, 567–570. [Google Scholar] [CrossRef]
- Sikarwar, M.S.; Patil, M.B. Antihyperlipidemic activity of Salacia chinensis root extracts in Triton-induced and atherogenic diet-induced hyperlipidemic rats. Indian J. Pharmacol. 2012, 44, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Compound | Inhibition of Lipid Peroxidation: IC50 (μΜ) # |
|---|---|
| 1 | 260 |
| 2 | 43 |
| 3 | 169 |
| 4 | 680 |
| 5 | - |
| 6 | - |
| 7 | 36 |
| Trolox | 25 |
| Compound | MW | TPSA (Å2) | ClogP | H Acceptors | H Donors | Violations |
|---|---|---|---|---|---|---|
| 1 | 279.35 | 49.77 | 1.68 | 4 | 1 | 0 |
| 2 | 310.34 | 55.76 | 4.02 | 4 | 1 | 0 |
| 3 | 309.38 | 59.00 | 1.46 | 5 | 1 | 0 |
| 4 | 340.37 | 64.99 | 3.80 | 5 | 1 | 0 |
| 5 | 293.38 | 38.77 | 2.16 | 4 | 0 | 0 |
| 6 | 324.37 | 44.76 | 4.50 | 4 | 0 | 0 |
| 7 | 576.12 | 76.07 | 9.14 | 6 | 1 | 2 |
| Compound | Percent Scavenging of DPPH (200 μΜ) | ||
|---|---|---|---|
| 200 μΜ | 100 μΜ | 50 μΜ | |
| 1 | 55.6 | 40.2 | 21.3 |
| 3 | 80.0 | 51.2 | 32.3 |
| 7 | 85.2 | 67.9 | 40.3 |
| Trolox | 92.0 | 90.0 | 38.0 |
| Compound | x% Edema Reduction |
|---|---|
| 1 | 53.0 ** |
| 2 | 25.0 * |
| 3 | 31.0 * |
| 4 | 17.0 * |
| 5 | 32.8 ** |
| 6 | 22.7 * |
| 7 | 72.0 ** |
| Ibuprofen | 36.0 * |
| Tolfenamic acid | 24.0 ** |
| Indomethacin | 42.0 ** |
| Compound | % Inhibition |
|---|---|
| 1 | 20 |
| 2 | 56 |
| 3 | - |
| 4 | - |
| 5 | 41 |
| 6 | 49 |
| 7 | 81 |
| Indomethacin | - |
| NDGA | 94 |
| Compound | % Reduction | ||
|---|---|---|---|
| Dose (i.p.) (μmol/kg) | TC a | TG b | |
| 1 | 150 | 72.6 ** | 70.3 * |
| 2 | 150 | 66.4 ** | 43.2 *** |
| 3 | 150 | 68.7 *** | 64.5 *** |
| 5 | 150 | 76.0 *** | 72.5 *** |
| 6 | 150 | 46.3 *** | 49.5 *** |
| Simvastatin | 150 | 73.0 *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants 2023, 12, 1436. https://doi.org/10.3390/antiox12071436
Theodosis-Nobelos P, Papagiouvannis G, Rekka EA. Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants. 2023; 12(7):1436. https://doi.org/10.3390/antiox12071436
Chicago/Turabian StyleTheodosis-Nobelos, Panagiotis, Georgios Papagiouvannis, and Eleni A. Rekka. 2023. "Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality" Antioxidants 12, no. 7: 1436. https://doi.org/10.3390/antiox12071436
APA StyleTheodosis-Nobelos, P., Papagiouvannis, G., & Rekka, E. A. (2023). Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants, 12(7), 1436. https://doi.org/10.3390/antiox12071436