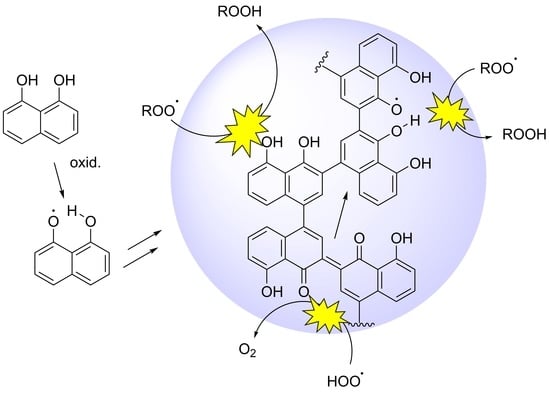
Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Vis Absorption Spectroscopy
2.3. Dynamic Light Scattering (DLS)
2.4. Transmission Electron Microscopy (TEM)
2.5. Electron Paramagnetic Resonance (EPR)
2.6. AOX Properties
2.6.1. Autoxidation of Tetrahydrofuran (THF) in Water at pH 7.4 [50]
2.6.2. Autoxidation of Styrene and γ-Terpinene in Acetonitrile [50]
2.7. Synthesis of PDHN
2.8. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Kinetic Studies of the Reaction with ROO• and HOO•
3.3. Mechanism of the Reaction with HOO•
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Escala, A. Universal relation for life-span energy consumption in living organisms: Insights for the origin of aging. Sci. Rep. 2022, 12, 2407. [Google Scholar] [CrossRef]
- Speakman, J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005, 208, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Wang, H.; Ge, Z.; Zuo, T.; Chen, Q.; Liu, X.; Mou, S.; Fan, C.; Xie, Y.; Wang, L. Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets. Nano Lett. 2020, 20, 1447–1454. [Google Scholar] [CrossRef]
- Mu, X.; He, H.; Wang, J.; Long, W.; Li, Q.; Liu, H.; Gao, Y.; Ouyang, L.; Ren, Q.; Sun, S.; et al. Carbogenic Nanozyme with Ultrahigh Reactive Nitrogen Species Selectivity for Traumatic Brain Injury. Nano Lett. 2019, 19, 4527–4534. [Google Scholar] [CrossRef]
- Xu, L.; Xu, R.; Saw, P.E.; Wu, J.; Cheng, S.-X.; Xu, X. Nanoparticle-Mediated Inhibition of Mitochondrial Glutaminolysis to Amplify Oxidative Stress for Combination Cancer Therapy. Nano Lett. 2021, 21, 7569–7578. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO(.) to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. 2021, 60, 15220–15224. [Google Scholar] [CrossRef] [PubMed]
- Mollica, F.; Lucernati, R.; Amorati, R. Expanding the spectrum of polydopamine antioxidant activity by nitroxide conjugation. J. Mater. Chem. B 2021, 9, 9980–9988. [Google Scholar] [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants—The fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Shang, Q.; Wu, J.; Zhang, H.; Liu, C.; Liu, K. Supramolecular Self-Assemblies with Self-Supplying H2O2 and Self-Consuming GSH Property for Amplified Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 37424–37435. [Google Scholar] [CrossRef]
- Daniels, R.; Morato, E.O.; Yassin, O.A.; Mao, J.; Mutlu, Z.; Jain, M.; Valenti, J.; Cakmak, M.; Nair, L.S.; Sotzing, G.A. Poly(cannabinoid)s: Hemp-Derived Biocompatible Thermoplastic Polyesters with Inherent Antioxidant Properties. ACS Appl. Mater. Interfaces 2022, 14, 42804–42811. [Google Scholar] [CrossRef]
- Han, Z.; Chen, S.; Deng, L.; Liang, Q.; Qu, X.; Li, J.; Wang, B.; Wang, H. Anti-Fouling, Adhesive Polyzwitterionic Hydrogel Electrodes Toughened Using a Tannic Acid Nanoflower. ACS Appl. Mater. Interfaces 2022, 14, 45954–45965. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, L.; Sun, H.; Wu, D.; Wang, C.; Ren, X.; Shao, Q.; York, P.; Tong, J.; Zhu, J.; et al. Antioxidant Biodegradable Covalent Cyclodextrin Frameworks as Particulate Carriers for Inhalation Therapy against Acute Lung Injury. ACS Appl. Mater. Interfaces 2022, 14, 38421–38435. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Shi, L.; Tang, Q.; Li, B.; Wang, X.; Jin, Y. Nanotrains of DNA Copper Nanoclusters That Triggered a Cascade Fenton-Like Reaction and Glutathione Depletion to Doubly Enhance Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 37280–37290. [Google Scholar] [CrossRef]
- Miller, R.; Kim, Y.; Park, C.G.; Torres, C.; Kim, B.; Lee, J.; Flaherty, D.; Han, H.-S.; Kim, Y.J.; Kong, H. Extending the Bioavailability of Hydrophilic Antioxidants for Metal Ion Detoxification via Crystallization with Polysaccharide Dopamine. ACS Appl. Mater. Interfaces 2022, 14, 39759–39774. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, B.; Ye, Z.; Zhang, W.; Xu, W.; Gao, S.; Zhou, N.; Wu, F.; Shen, J. Enzyme-Responsive COF-Based Thiol-Targeting Nanoinhibitor for Curing Bacterial Infections. ACS Appl. Mater. Interfaces 2022, 14, 38483–38496. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, X.; Li, Z.; Wang, Z.; Luo, Y.; Deng, L.; He, D. Ultrastretchable, Self-Healable, and Tissue-Adhesive Hydrogel Dressings Involving Nanoscale Tannic Acid/Ferric Ion Complexes for Combating Bacterial Infection and Promoting Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 43010–43025. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Li, M.; Li, Z.; Zhang, H.; Guo, B.; Zhang, J. Antibacterial Conductive UV-Blocking Adhesion Hydrogel Dressing with Mild On-Demand Removability Accelerated Drug-Resistant Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 41726–41741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Adhikari, B.; Wang, H. Preparation of a Novel Carbon Dot/Polyvinyl Alcohol Composite Film and Its Application in Food Preservation. ACS Appl. Mater. Interfaces 2022, 14, 37528–37539. [Google Scholar] [CrossRef]
- Xu, J.; Chu, T.; Yu, T.; Li, N.; Wang, C.; Li, C.; Zhang, Y.; Meng, H.; Nie, G. Design of Diselenide-Bridged Hyaluronic Acid Nano-antioxidant for Efficient ROS Scavenging to Relieve Colitis. ACS Nano 2022, 16, 13037–13048. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Z.; Jia, B.; Shi, Y.; Dong, J.; Jiang, S.; Li, Z. DNA Origami as a Nanomedicine for Targeted Rheumatoid Arthritis Therapy through Reactive Oxygen Species and Nitric Oxide Scavenging. ACS Nano 2022, 16, 12520–12531. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Scurti, S.; Caretti, D.; Mollica, F.; Di Antonio, E.; Amorati, R. Chain-Breaking Antioxidant and Peroxyl Radical Trapping Activity of Phenol-Coated Magnetic Iron Oxide Nanoparticles. Antioxidants 2022, 11, 1163. [Google Scholar] [CrossRef]
- Viglianisi, C.; Scarlini, A.; Tofani, L.; Menichetti, S.; Baschieri, A.; Amorati, R. Magnetic nanoantioxidants with improved radical-trapping stoichiometry as stabilizers for inhibition of peroxide formation in ethereal solvents. Sci. Rep. 2019, 9, 17219. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Polak-Śliwińska, M.; Kowalczyk, E.; Sienkiewicz, M. Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol. Polymers 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Manini, P.; Martins, Z.; Remusat, L.; O’D. Alexander, C.M.; Puzzarini, C.; Barone, V.; Saladino, R. Insoluble organic matter in chondrites: Archetypal melanin-like PAH-based multifunctionality at the origin of life? Phys. Life Rev. 2021, 37, 65–93. [Google Scholar] [CrossRef]
- Singla, S.; Htut, K.Z.; Zhu, R.; Davis, A.; Ma, J.; Ni, Q.Z.; Burkart, M.D.; Maurer, C.; Miyoshi, T.; Dhinojwala, A. Isolation and Characterization of Allomelanin from Pathogenic Black Knot Fungus—A Sustainable Source of Melanin. ACS Omega 2021, 6, 35514–35522. [Google Scholar] [CrossRef]
- Lino, V.; Manini, P. Dihydroxynaphthalene-Based Allomelanins: A Source of Inspiration for Innovative Technological Materials. ACS Omega 2022, 7, 15308–15314. [Google Scholar] [CrossRef]
- Tsouko, E.; Tolia, E.; Sarris, D. Microbial Melanin: Renewable Feedstock and Emerging Applications in Food-Related Systems. Sustainability 2023, 15, 7516. [Google Scholar] [CrossRef]
- Argenziano, R.; Alfieri, M.L.; Arntz, Y.; Castaldo, R.; Liberti, D.; Maria Monti, D.; Gentile, G.; Panzella, L.; Crescenzi, O.; Ball, V.; et al. Non-covalent small molecule partnership for redox-active films: Beyond polydopamine technology. J. Colloid Interface Sci. 2022, 624, 400–410. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. The Photophysics and Photochemistry of Melanin- Like Nanomaterials Depend on Morphology and Structure. Chemistry 2021, 27, 16309–16319. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Manini, P.; Lino, V.; Franchi, P.; Gentile, G.; Sibillano, T.; Giannini, C.; Picardi, E.; Napolitano, A.; Valgimigli, L.; Chiappe, C.; et al. A Robust Fungal Allomelanin Mimic: An Antioxidant and Potent π-Electron Donor with Free-Radical Properties that can be Tuned by Ionic Liquids. ChemPlusChem 2019, 84, 1331–1337. [Google Scholar] [CrossRef]
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Zhu, H.; Zhu, J.; Wang, Z.; Xing, Y.; Xie, X.; Cai, K.; Zhang, J. Nanomodulators Capable of Timely Scavenging ROS for Inflammation and Prognosis Control Following Photothermal Tumor Therapy. Adv. Funct. Mater. 2023, 33, 2213151. [Google Scholar] [CrossRef]
- McCallum, N.C.; Son, F.A.; Clemons, T.D.; Weigand, S.J.; Gnanasekaran, K.; Battistella, C.; Barnes, B.E.; Abeyratne-Perera, H.; Siwicka, Z.E.; Forman, C.J.; et al. Allomelanin: A Biopolymer of Intrinsic Microporosity. J. Am. Chem. Soc. 2021, 143, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants 2021, 10, 1551. [Google Scholar] [CrossRef]
- Baschieri, A.; Jin, Z.; Amorati, R. Hydroperoxyl radical (HOO•) as a reducing agent: Unexpected synergy with antioxidants. A Review. Free Radic. Res. 2023, 57, 115–129. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chemistry 2016, 22, 7924–7934. [Google Scholar] [CrossRef]
- Shah, R.; Poon, J.-F.; Haidasz, E.A.; Pratt, D.A. Temperature-Dependent Effects of Alkyl Substitution on Diarylamine Antioxidant Reactivity. J. Org. Chem. 2021, 86, 6538–6550. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Bietti, M.; Galeotti, M.; Salamone, M.; Lanzalunga, O.; Cecchini, M.M.; Reale, S.; Crescenzi, O.; Napolitano, A.; De Angelis, F.; et al. Characterization and Fate of Hydrogen-Bonded Free-Radical Intermediates and Their Coupling Products from the Hydrogen Atom Transfer Agent 1,8-Naphthalenediol. ACS Omega 2018, 3, 3918–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Guerra, M.; Amorati, R.; Pedulli, G.F. Water Effect on the O−H Dissociation Enthalpy of Para-Substituted Phenols: A DFT Study. J. Org. Chem. 2004, 69, 5460–5467. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Cabiddu, S.; Fattuoni, C. Bond Dissociation Energies of O−H Bonds in Substituted Phenols from Equilibration Studies. J. Org. Chem. 1996, 61, 9259–9263. [Google Scholar] [CrossRef]
- Mulder, P.; Korth, H.G.; Pratt, D.A.; DiLabio, G.A.; Valgimigli, L.; Pedulli, G.F.; Ingold, K.U. Critical re-evaluation of the O-H bond dissociation enthalpy in phenol. J. Phys. Chem. A 2005, 109, 2647–2655. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Gong, X.; Cao, W.; Forman, C.J.; Oktawiec, J.; D’Alba, L.; Sun, H.; Thompson, M.P.; Hu, Z.; Kapoor, U.; et al. Anisotropic Synthetic Allomelanin Materials via Solid-State Polymerization of Self-Assembled 1,8-Dihydroxynaphthalene Dimers. Angew. Chem. 2021, 60, 17464–17471. [Google Scholar] [CrossRef]
- Foti, M.C.; Johnson, E.R.; Vinqvist, M.R.; Wright, J.S.; Barclay, L.R.C.; Ingold, K.U. Naphthalene Diols: A New Class of Antioxidants Intramolecular Hydrogen Bonding in Catechols, Naphthalene Diols, and Their Aryloxyl Radicals. J. Org. Chem. 2002, 67, 5190–5196. [Google Scholar] [CrossRef] [Green Version]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L. Kinetic and thermodynamic aspects of the chain-breaking antioxidant activity of ascorbic acid derivatives in non-aqueous media. Org. Biomol. Chem. 2011, 9, 3792–3800. [Google Scholar] [CrossRef]
- Cedrowski, J.; Litwinienko, G.; Baschieri, A.; Amorati, R. Hydroperoxyl Radicals (HOO.): Vitamin E Regeneration and H-Bond Effects on the Hydrogen Atom Transfer. Chemistry 2016, 22, 16441–16445. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of Lipid Peroxidation by γ-Terpinene, an Unusual and Potentially Useful Hydrocarbon Antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Poon, J.-F.; Zilka, O.; Pratt, D.A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331–14342. [Google Scholar] [CrossRef]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO· Radicals and Implication for Catalytic Coantioxidant Systems. J. Am. Chem. Soc. 2018, 140, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Shah, R.; Van Kessel, A.T.; Zilka, O.; Haidasz, E.A.; Pratt, D.A. The Catalytic Reaction of Nitroxides with Peroxyl Radicals and Its Relevance to Their Cytoprotective Properties. J. Am. Chem. Soc. 2018, 140, 3798–3808. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Methods To Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Cecchini, M.M.; Reale, S.; Manini, P.; d’Ischia, M.; De Angelis, F. Modeling Fungal Melanin Buildup: Biomimetic Polymerization of 1,8-Dihydroxynaphthalene Mapped by Mass Spectrometry. Chemistry 2017, 23, 8092–8098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, F.; Hamarat, B.; Ahmadli, D.; Şahin, O.; Bozkaya, U.; Türkmen, Y.E. Polarization-Enhanced Hydrogen Bonding in 1,8-Dihydroxynaphthalene: Conformational Analysis, Binding Studies and Hydrogen Bonding Catalysis. ChemistrySelect 2020, 5, 13387–13396. [Google Scholar] [CrossRef]
- Manini, P.; Lino, V.; D’Errico, G.; Reale, S.; Napolitano, A.; De Angelis, F.; d’Ischia, M.J.P.C. “Blackness” is an index of redox complexity in melanin polymers. Polym. Chem. 2020, 11, 5005–5010. [Google Scholar] [CrossRef]
- Wang, J.; Blancafort, L.J.A.C. Stability and optical absorption of a comprehensive virtual library of minimal eumelanin oligomer models. Angew. Chem. 2021, 133, 18948–18957. [Google Scholar] [CrossRef]
- Chen, C.-T.; Martin-Martinez, F.J.; Jung, G.S.; Buehler, M.J.J.C.s. Polydopamine and eumelanin molecular structures investigated with ab initio calculations. Chem. Sci. 2017, 8, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as Neuroprotective Agents. Antioxidants 2023, 12, 1464. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridi-Printezi, A.; Mollica, F.; Lucernati, R.; Montalti, M.; Amorati, R. Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles. Antioxidants 2023, 12, 1511. https://doi.org/10.3390/antiox12081511
Mavridi-Printezi A, Mollica F, Lucernati R, Montalti M, Amorati R. Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles. Antioxidants. 2023; 12(8):1511. https://doi.org/10.3390/antiox12081511
Chicago/Turabian StyleMavridi-Printezi, Alexandra, Fabio Mollica, Rosa Lucernati, Marco Montalti, and Riccardo Amorati. 2023. "Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles" Antioxidants 12, no. 8: 1511. https://doi.org/10.3390/antiox12081511
APA StyleMavridi-Printezi, A., Mollica, F., Lucernati, R., Montalti, M., & Amorati, R. (2023). Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles. Antioxidants, 12(8), 1511. https://doi.org/10.3390/antiox12081511