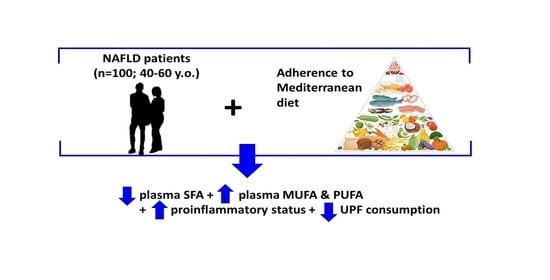
Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Methods
2.1. Design and Participants
2.2. Adherence to the Mediterranean Diet
2.3. Anthropometrics and Blood Pressure
2.4. Biochemical Parameters
2.5. Dietary Inflammatory Index
2.6. Dietary Intake Assessment
2.7. Oxidative Stress and Inflammatory Biomarkers
2.8. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis of Fatty Acids
2.9. Statistics
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Llompart, I.; Gámez, J.M.; Tejada, S.; Martínez, J.A.; et al. A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; Lavine, J.E.; Ratziu, V.; Mccullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, P.; Byrne, C.D. Bidirectional Relationships and Disconnects between NAFLD and Features of the Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelber-Sagi, S.; Godos, J.; Salomone, F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: A review of observational studies and intervention trials. Therap. Adv. Gastroenterol. 2016, 9, 392–407. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Burgos Peláez, R.; Rivera Irigoin, R. ESPEN Practical Guideline: Clinical nutrition in liver disease. Nutr. Hosp. 2022, 39, 434–472. [Google Scholar] [CrossRef]
- Hydes, T.J.; Ravi, S.; Loomba, R.; Gray, M.E. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 2020, 26, 383–400. [Google Scholar] [CrossRef]
- Musazadeh, V.; Karimi, A.; Malekahmadi, M.; Ahrabi, S.S.; Dehghan, P. Omega-3 polyunsaturated fatty acids in the treatment of non-alcoholic fatty liver disease: An umbrella systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 327–334. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Vázquez, C.; Botella-Carretero, J.I.; Corella, D.; Fiol, M.; Lage, M.; Lurbe, E.; Richart, C.; Fernández-Real, J.M.; Fuentes, F.; Ordóñez, A.; et al. White fish reduces cardiovascular risk factors in patients with metabolic syndrome: The WISH-CARE study, a multicenter randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 328–335. [Google Scholar] [CrossRef]
- Konieczna, J.; Fiol, M.; Colom, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Soria-Florido, M.T.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Does Consumption of Ultra-Processed Foods Matter for Liver Health? Prospective Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 4142. [Google Scholar] [CrossRef]
- Konieczna, J.; Morey, M.; Abete, I.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Vioque, J.; Gonzalez-Palacios, S.; Daimiel, L.; Salas-Salvadó, J.; Fiol, M.; et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: Prospective analysis nested in the PREDIMED-Plus trial. Clin. Nutr. 2021, 40, 4290–4300. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Ó.; Guzmán-Lafuente, P.; Serna, J.; Muñoz-Hernández, M.; López Neyra, A.; García-Rozas, P.; García-Seisdedos, D.; Alcázar, A.; Lasunción, M.A.; Busto, R.; et al. A comprehensive evaluation of omega-3 fatty acid supplementation in cystic fibrosis patients using lipidomics. J. Nutr. Biochem. 2019, 63, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Llompart, I.; Ugarriza, L.; Martínez, J.A.; Tur, J.A.; et al. Increased Adherence to the Mediterranean Diet after Lifestyle Intervention Improves Oxidative and Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1440. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Prevention and Reversion of NAFLD in Obese Patients With Metabolic Syndrome by Mediterranean Diet and Physical Activity. Available online: https://clinicaltrials.gov/ct2/show/NCT04442620 (accessed on 8 February 2023).
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef] [Green Version]
- Eskreis-Winkler, S.; Corrias, G.; Monti, S.; Zheng, J.; Capanu, M.; Krebs, S.; Fung, M.; Reeder, S.; Mannelli, L. IDEAL-IQ in an oncologic population: Meeting the challenge of concomitant liver fat and liver iron. Cancer Imaging 2018, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- De La Iglesia, R.; Lopez-Legarrea, P.; Abete, I.; Bondia-Pons, I.; Navas-Carretero, S.; Forga, L.; Martinez, J.A.; Zulet, M.A. A new dietary strategy for long-term treatment of the metabolic syndrome is compared with the American heart association (AHA) guidelines: The MEtabolic Syndrome REduction in NAvarra (RESMENA) project. Br. J. Nutr. 2014, 111, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Alfaro, L.; Del Mar Bibiloni, M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvad, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente-Arrillaga, C.; Vzquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Food Composition Tables; Ediciones Pirámide: Madrid, Spain, 2018; p. 496. (In Spanish) [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarae, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright [Food classification. Public health]. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar]
- Lepage, G.; Roy, C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Abu, E.O.; Oluwatowoju, I. Omega-3 index determined by gas chromatography with electron impact mass spectrometry. Prostagland. Leukot. Essent. Fat. Acids 2009, 80, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.; Couture, P.; Lamarche, B. Diet Quality, Saturated Fat and Metabolic Syndrome. Nutrients 2020, 12, 3232. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart. 2018, 5, e000946. [Google Scholar] [CrossRef]
- Celada Roldan, C.; Tarraga Marcos, M.L.; Madrona Marcos, F.; Solera Albero, J.; Salmeron Rios, R.; Celada Rodriguez, A.; Panisello Royo, J.M.; Tárraga López, P.J. Adhesion to the Mediterranean diet in diabetic patients with poor control. Clin. Investig. Arterioscler. 2019, 31, 210–217. [Google Scholar] [CrossRef]
- Díez-Espino, J.; Buil-Cosiales, P.; Serrano-Martínez, M.; Toledo, E.; Salas-Salvadó, J.; Martínez-González, M.Á. Adherence to the Mediterranean diet in patients with type 2 diabetes mellitus and HbA1c level. Ann. Nutr. Metab. 2011, 58, 74–78. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.D.M.; Bouzas, C.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Total and Subtypes of Dietary Fat Intake and Its Association with Components of the Metabolic Syndrome in a Mediterranean Population at High Cardiovascular Risk. Nutrients 2019, 11, 1493. [Google Scholar] [CrossRef] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef]
- Maciejewska-Markiewicz, D.; Drozd, A.; Palma, J.; Ryterska, K.; Hawryłkowicz, V.; Załęska, P.; Wunsh, E.; Kozłowska-Petriczko, K.; Stachowska, E. Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients-Randomized Control Trials (RCT). Nutrients 2022, 14, 4310. [Google Scholar] [CrossRef]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.H.; Wang, Y.F.; Xu, Q.H.; Chen, S.S. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 516–521. [Google Scholar] [CrossRef]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A Low ω-6 to ω-3 PUFA Ratio (n-6:n-3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
- Kelley, N.S. Treatment of Nonalcoholic Fatty Liver Disease with Long-Chain n-3 Polyunsaturated Fatty Acids in Humans. Metab. Syndr. Relat. Disord. 2016, 14, 417–430. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [Green Version]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.D.M.; Tur, J.A. Dietary fat intake and metabolic syndrome in adults: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 887–905. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef]
- Harris, K.; Oshima, M.; Sattar, N.; Würtz, P.; Jun, M.; Welsh, P.; Hamet, P.; Harrap, S.; Poulter, N.; Chalmers, J.; et al. Plasma fatty acids and the risk of vascular disease and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE study. Diabetologia 2020, 63, 1637–1647. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Busquets-Cortés, C.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Resolvins as proresolving inflammatory mediators in cardiovascular disease. Eur. J. Med. Chem. 2018, 153, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L.A. Impact of the Co-Administration of N-3 Fatty Acids and Olive Oil Components in Preclinical Nonalcoholic Fatty Liver Disease Models: A Mechanistic View. Nutrients 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gormaz, J.G.; Rodrigo, R.; Videla, L.A.; Beems, M. Biosynthesis and bioavailability of long-chain polyunsaturated fatty acids in non-alcoholic fatty liver disease. Prog. Lipid Res. 2010, 49, 407–419. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Pettinelli, P.; Araya, A.V.; Poniachik, J.; Videla, L. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity 2010, 18, 1460–1463. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.Á.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar]
- Petermann-Rocha, F.; Wirth, M.D.; Boonpor, J.; Parra-Soto, S.; Zhou, Z.; Mathers, J.C.; Livingstone, K.; Forrest, E.; Pell, J.P.; Ho, F.K.; et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: A prospective study of 171,544 UK Biobank participants. BMC Med. 2023, 21, 123. [Google Scholar]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; García, S.; Mateos, D.; Gómez, C.; Gámez, J.M.; Poulsen, H.E.; Tur, J.A.; Sureda, A. Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk. Antioxidants 2022, 11, 1326. [Google Scholar] [PubMed]
- Gorska, P.; Gorna, I.; Przyslawski, J. Mediterranean diet and oxidative stress. Nutr. Food Sci. 2021, 51, 677–689. [Google Scholar]
- Chatzianagnostou, K.; Del Turco, S.; Pingitore, A.; Sabatino, L.; Vassalle, C. The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging. Antioxidants 2015, 4, 719–736. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar]


| Group 1: Unprocessed or minimally processed foods | Milk (whole-fat, semi-skimmed, and skimmed), yogurt (whole-fat and skimmed), eggs, meats (chicken, turkey, beef, pork, lamb, rabbit), liver, offal, fish and seafoods, fresh vegetables, gazpacho, boiled potatoes, fresh fruits, dried fruits, nuts, legumes, whole-grain cereals, rice (whole-grain and refined), pasta (whole-grain and refined), natural fruit juices, coffee, and tea. |
| Group 2: Processed culinary ingredients | Vegetable oils (regular and virgin extra olive oil, oils from sunflower seeds, corn, and soybeans), butter, lard, salt, sugar, and honey. |
| Group 3: Processed foods | Condensed milk, cream, cheeses (cured, semi-cured, cottage, and fresh), bacon, cured ham, canned fish, home-made French fries, olives, fruits in syrup, breads (white and whole grain), marmalade, decaffeinated coffee, beer, wine, and champagne. |
| Group 4: Ultra-processed foods | Milkshakes, petit-suisse, custard, ice cream, processed meat (ham, chorizo, mortadella, sausages, hamburgers, meat balls, pate), potato chips, breakfast cereals, cookies, industrial and commercial pastries (croissants, donuts, muffins, cakes, churros), chocolates, sugary cocoa powder, marzipan, nougat, creamy cheese spreads, margarine, pre-prepared dishes (croquettes, pizza), instant soups, mayonnaise, mustard, ketchup, packed fried tomato sauce, savoury packed snacks, commercial fruit juices, alcoholic drinks produced by fermentation followed by distillation (liquors, whisky, vodka, gin, liquors). |
| AMD < 7 n = 30 | AMD 7–9 n = 35 | AMD > 9 n = 35 | p-Value | |
|---|---|---|---|---|
| Sex (%) | ||||
| Men | 56.5 | 56.0 | 45.5 | 0.782 |
| Women | 43.5 | 44.0 | 54.5 | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 50.1 (6.8) | 51.8 (7.8) | 54.8 (5.9) * | 0.007 |
| Weight (kg) | 93.9 (12.3) | 92.3 (10.4) | 90.3 (8.6) | 0.473 |
| BMI (kg/m2) | 32.7 (2.8) | 32.5 (3.9) | 33.4 (2.8) | 0.336 |
| Systolic blood pressure (mmHg) | 136.8 (13.4) | 135.9 (10.6) | 135.3 (14.1) | 0.857 |
| Diastolic blood pressure (mmHg) | 82.4 (8.0) | 82.9 (7.0) | 81.6 (6.3) | 0.652 |
| Glucose (mg/dL) | 117.2 (63.1) | 105.5 (21.8) | 110.5 (32.6) | 0.475 |
| HBA1c (%) | 6.08 (1.69) | 5.80 (0.81) | 6.01 (1.19) | 0.572 |
| Triglycerides (mg/dL) | 217.1 (121.4) | 158.1 (77.7) * | 151.4 (69.3) * | 0.006 |
| HDL-cholesterol (mg/dL) | 42.1 (7.7) | 45.9 (13.6) | 45.7 (8.2) | 0.255 |
| LDL-cholesterol (mg/dL) | 135.1 (26.3) | 133.5 (36.4) | 127.6 (31.9) | 0.588 |
| Total cholesterol (mg/dL) | 223.7 (41.4) | 217.4 (59.7) | 203.4 (35.3) | 0.190 |
| AST (U/L) | 24.5 (10.0) | 24.6 (12.8) | 22.9 (7.07) | 0.775 |
| ALT (U/L) | 31.1 (17.4) | 27.7 (11.5) | 25.7 (10.5) | 0.284 |
| GGT (U/L) | 42.4 (23.6) | 31.9 (14.3) * | 33.3 (14.1) * | 0.048 |
| CRP (mg/dL) | 0.570 (0.841) | 0.489 (0.554) | 0.468 (0.458) | 0.776 |
| Bilirubin total (mg/dL) | 0.713 (0.411) | 0.717 (0.294) | 0.689 (0.346) | 0.934 |
| IFC (%) | 13.1 (9.0) | 12.8 (9.9) | 12.0 (8.59) | 0.869 |
| DII | 0.751 (2.1) | 0.559 (2.5) | −0.693 (2.1) *# | 0.011 |
| AMD < 7 n = 30 | AMD 7–9 n = 35 | AMD > 9 n = 35 | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Fruits (g/day) | 217.3 (199.3) | 299.4 (199.7) | 372.5 (213.6) * | 0.032 |
| Vegetables (g/day) | 284.9 (187.8) | 321.3 (193.8) | 351.9 (190.2) | 0.302 |
| Extra virgin olive oil (g/day) | 14.4 (16.1) | 17.7 (3.4) | 17.4 (3.5) | 0.079 |
| Fat from foods of animal origin (g/day) | 49.1 (24.3) | 45.7 (16.1) | 42.7 (18.5) | 0.544 |
| Plant-based fat (g/day) | 54.2 (21.4) | 57.0 (18.5) | 63.3 (33.1) | 0.471 |
| MUFA (g/day) | 48.7 (21.5) | 47.3 (13.8) | 50.9 (20.6) | 0.786 |
| PUFAs (g/day) | 17.5 (7.7) | 16.2 (5.3) | 18.3 (11.7) | 0.693 |
| SFAs (g/day) | 27.2 (12.1) | 26.4 (7.6) | 26.2 (10.7) | 0.938 |
| Cholesterol (mg/day) | 445.4 (178.6) | 378.8 (115.8) | 375.7 (134.6) | 0.205 |
| Phytosterols (mg/day) | 331.7 (95.9) | 325.4 (91.3) | 382.9 (136.3) | 0.139 |
| Linoleic acid (g/day) | 12.5 (6.0) | 11.1 (3.9) | 11.5 (5.4) | 0.679 |
| Linolenic acid (g/day) | 1.17 (0.54) | 1.18 (0.42) | 1.35 (0.77) | 0.505 |
| Arachidonic acid (g/day) | 0.130 (0.059) | 0.151 (0.067) | 0.138 (0.065) | 0.423 |
| Omega-3 EPA + DPA + DHA (g/day) | 0.575 (0.331) | 0.599 (0.326) | 0.976 (0.651) *# | 0.009 |
| Omega-3 from fish + EPA+ DHA (g/day) | 0.372 (0.165) | 0.570 (0.393) | 0.746 (0.511) * | 0.016 |
| Omega-3 non-animal origin (g/day) | 0.116 (0.060) | 0.093 (0.045) | 0.082 (0.045) | 0.083 |
| Ratio n-6:n-3 | 5.98 (1.87) | 4.88 (1.52) | 4.17 (1.82) * | 0.013 |
| Trans-fatty acids (g/day) | 0.729 (0.451) | 0.577 (0.205) | 0.564 (0.295) | 0.189 |
| Unprocessed or minimally processed foods (% of consumption) | 59.8 (16.9) | 67.6 (13.9) | 72.5 (11.5) * | 0.013 |
| Processed culinary ingredients (% of consumption) | 2.1 (1.0) | 2.2 (1.3) | 2.6 (1.8) | 0.471 |
| Processed foods (% of consumption) | 15.9 (9.4) | 15.8 (8.7) | 14.4 (5.3) | 0.776 |
| Ultra-processed food (% of consumption) | 33.3 (20.7) | 14.6 (13.3) * | 12.7 (16.5) * | <0.001 |
| AMD < 7 n = 30 | AMD 7–9 n = 35 | AMD > 9 n = 35 | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| SFAs | ||||
| Myristic acid, C14:0 (nM) | 110.1 (34.8) | 77.3 (21.9) * | 79.4 (21.7) * | <0.001 |
| Palmitic acid, C16:0 (nM) | 1651.3 (331.8) | 1410.0 (215.8) * | 1477.3 (278.2) * | <0.001 |
| Stearic acid, C18:0 (nM) | 640.3 (73.7) | 592.6 (65.7) * | 585.1 (64.3) * | 0.005 |
| Arachidic acid, C20:0 (nM) | 13.9 (1.9) | 13.4 (1.3) | 13.8 (1.5) | 0.413 |
| Docosanoic acid, C22:0 (nM) | 30.2 (5.1) | 27.1 (4.3) * | 27.1 (4.9) * | 0.014 |
| Lignoceric acid, C24:0 (nM) | 31.1(7.5) | 27.1 (7.0) | 26.0 (5.9) * | 0.010 |
| MUFA | ||||
| Palmitoleic acid, C16:1 (nM) | 120.3 (45.5) | 95.6 (43.4) * | 83.6 (26.9) * | 0.002 |
| n-9 PUFA | ||||
| Oleic acid, C18:1 n9 cis/trans (nM) | 1228.6 (445.1) | 1060.8 (304.4) | 1088.7 (348.4) | 0.133 |
| n-6 PUFAs | ||||
| Linoleic acid, C18:2 n6, LA (nM) | 1724.9 (326.5) | 1352.3 (272.3) * | 1389.3 (211.2) * | <0.001 |
| Gamma-linoleic acid, C18:3 n6 (nM) | 35.7 (13.0) | 26.0 (8.3) * | 24.3 (10.5) * | <0.001 |
| Dihomo-y-linoleic acid C20:3 n6 (nM) | 118.1 (24.8) | 99.6 (27.0) * | 96.3 (27.0) * | 0.004 |
| Arachidonic acid, C20:4 n6 (nM) | 464.9 (129.1) | 443.5 (122.2) | 414.6 (119.8) | 0.265 |
| Adrenic acid, C22:4 n6 (nM) | 21.0 (7.8) | 15.1 (5.0) * | 13.9 (5.1) * | <0.001 |
| Docosapentaenoic acid (DPA), C22:5 n6 (nM) | 16.4 (6.6) | 14.4 (6.1) * | 11.3 (4.1) * | 0.007 |
| n-3 PUFAs | ||||
| Alfa-linoleic acid (ALA), C18:3 n3 (nM) | 19.8 (6.0) | 18.4 (6.4) | 19.7 (6.1) | 0.568 |
| Eicosapentaenoic acid (EPA), C20:5 n3 (nM) | 25.0 (12.1) | 32.8 (14.2) | 37.0 (17.5) * | 0.008 |
| Docosapentaenoic acid (DPA), C22:5 n3 (nM) | 25.8 (7.1) | 25.3 (6.2) | 26.2 (7.0) | 0.833 |
| Docosahexaeonic acid (DHA), C22:6 n3 (nM) | 118.1 (32.2) | 123.4 (34.7) | 127.7 (27.0) | 0.495 |
| FAME Total (nM) | 6521.6 (1090.1) | 5757.1 (966.0) * | 5749.6 (1021.9) * | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Bouzas, C.; Pastor, O.; Ugarriza, L.; Llompart, I.; Cevallos-Ibarra, K.; Sureda, A.; Tur, J.A. Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2023, 12, 1554. https://doi.org/10.3390/antiox12081554
Monserrat-Mesquida M, Quetglas-Llabrés MM, Bouzas C, Pastor O, Ugarriza L, Llompart I, Cevallos-Ibarra K, Sureda A, Tur JA. Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants. 2023; 12(8):1554. https://doi.org/10.3390/antiox12081554
Chicago/Turabian StyleMonserrat-Mesquida, Margalida, Maria Magdalena Quetglas-Llabrés, Cristina Bouzas, Oscar Pastor, Lucía Ugarriza, Isabel Llompart, Karla Cevallos-Ibarra, Antoni Sureda, and Josep A. Tur. 2023. "Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease" Antioxidants 12, no. 8: 1554. https://doi.org/10.3390/antiox12081554
APA StyleMonserrat-Mesquida, M., Quetglas-Llabrés, M. M., Bouzas, C., Pastor, O., Ugarriza, L., Llompart, I., Cevallos-Ibarra, K., Sureda, A., & Tur, J. A. (2023). Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants, 12(8), 1554. https://doi.org/10.3390/antiox12081554