Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice
Abstract
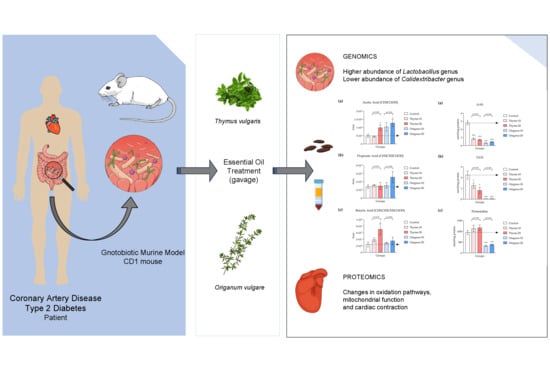
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Animals
2.2. Humanized Gnotobiotic Mouse Model
2.3. Essential Oil Composition and Preparation of Essential Oil Emulsions and Vehicle
2.4. Design and Experimental Groups
2.5. Metagenomic Analysis of Gut Microbiota in Feces
2.6. Determination of SCFA Levels in Feces
2.7. Proteomic Analysis of Cardiac Tissues
2.8. Determination of Carbonyls and Pentosidine in Plasma
2.9. Determination of Metabolites Related to Cardiovascular Health in Plasma
2.10. Statistical Analysis
3. Results
3.1. Alterations in Bacterial Abundance of Gut Microbiota Following Essential Oil Treatments
3.2. Elevated SCFA Levels Confirm the Favorable Impact of Essential Oils on Gut Microbiota
3.3. Over-Representation of Pathways Associated with Cardiac Contraction and Mitochondrial Function in Lower Abundance
3.4. Decline of Protein Oxidation and Glycoxidation in Plasma
3.5. Decreased Levels in TMAO and Cholesterol in Plasma as Indicators of Cardiovascular Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saljoughian, S.; Roohinejad, S.; Bekhit, A.E.D.A.; Greiner, R.; Omidizadeh, A.; Nikmaram, N.; Mousavi Khaneghah, A. The Effects of Food Essential Oils on Cardiovascular Diseases: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1688–1705. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Cantin, J.; Nigam, A. Contemporary Issues Regarding Nutrition in Cardiovascular Rehabilitation. Ann. Phys. Rehabil. Med. 2017, 60, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Padilha, G.; Sanches Machado d’Almeida, K.; Ronchi Spillere, S.; Corrêa Souza, G. Dietary Patterns in Secondary Prevention of Heart Failure: A Systematic Review. Nutrients 2018, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Bentham Science Publisher, B.S.P. The Gut Microbiota and Lipid Metabolism: Implications for Human Health and Coronary Heart Disease. Curr. Med. Chem. 2006, 13, 3005–3021. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Martin, M.; de la Vega-Correa, L.; Zapata, C.; Burokas, A.; Blasco, G.; Coll, C.; Escrichs, A.; et al. Microbiota Alterations in Proline Metabolism Impact Depression. Cell Metab. 2022, 34, 681–701.e10. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Metabolism and Nutrition: Effect of Thymol and Carvacrol Feed Supplementation on Performance, Antioxidant Enzyme Activities, Fatty Acid Composition, Digestive Enzyme Activities, and Immune Response in Broiler Chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef]
- Alagawany, M. Biological Effects and Modes of Action of Carvacrol in Animal and Poultry Production and Health—A Review. Adv. Anim. Vet. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X.G. The Effect of Essential Oils on Performance, Immunity and Gut Microbial Population in Weaner Pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of Acetate, Propionate, and Butyrate Formation by the Human Fecal Microbial Flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The Short-Chain Fatty Acid Pentanoate Suppresses Autoimmunity by Modulating the Metabolic-Epigenetic Crosstalk in Lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Sánchez-Quintero, M.J.; Delgado, J.; Medina-Vera, D.; Becerra-Muñoz, V.M.; Queipo-Ortuño, M.I.; Estévez, M.; Plaza-Andrades, I.; Rodríguez-Capitán, J.; Sánchez, P.L.; Crespo-Leiro, M.G.; et al. Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 4650. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Bețiu, A.M.; Noveanu, L.; Hâncu, I.M.; Lascu, A.; Petrescu, L.; Maack, C.; Elmér, E.; Muntean, D.M. Mitochondrial Effects of Common Cardiovascular Medications: The Good, the Bad and the Mixed. Int. J. Mol. Sci. 2022, 23, 13653. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Luna, C.; Arjona, A.; Dueñas, C.; Estevez, M. Allysine and α-Aminoadipic Acid as Markers of the Glyco-Oxidative Damage to Human Serum Albumin under Pathological Glucose Concentrations. Antioxidants 2021, 10, 474. [Google Scholar] [CrossRef]
- Sell, D.R.; Strauch, C.M.; Shen, W.; Monnier, V.M. 2-Aminoadipic Acid Is a Marker of Protein Carbonyl Oxidation in the Aging Human Skin: Effects of Diabetes, Renal Failure and Sepsis. Biochem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural Products as a Source of Inspiration for Novel Inhibitors of Advanced Glycation Endproducts (AGEs) Formation. Planta Medica 2021, 87, 780–801. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Ward, T.; Sidiropoulos, D.; Hillmann, B.M.; Chun, C.L.; Sadowsky, M.J.; Knights, D.; Montassier, E. Fecal Microbiota Transplantation Reverses Antibiotic and Chemotherapy-Induced Gut Dysbiosis in Mice. Sci. Rep. 2018, 8, 6219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut Microbiota from NLRP3-Deficient Mice Ameliorates Depressive-like Behaviors by Regulating Astrocyte Dysfunction via CircHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal Microbiota Containing Barnesiella Species Cures Vancomycin-Resistant Enterococcus Faecium Colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Erofeev, A.; Sandvik, A.; Grcic, V.; Jahnsen, F.L.; Gaustad, P.; McCoy, K.D.; Macpherson, A.J.; Meza-Zepeda, L.A.; Johansen, F.E. Depletion of Murine Intestinal Microbiota: Effects on Gut Mucosa and Epithelial Gene Expression. PLoS ONE 2011, 6, e17996. [Google Scholar] [CrossRef]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Sanchez-Alcoholado, L.; Castellano-Castillo, D.; Jordán-Martínez, L.; Moreno-Indias, I.; Cardila-Cruz, P.; Elena, D.; Muñoz-Garcia, A.J.; Queipo-Ortuño, M.I.; Jimenez-Navarro, M. Role of Gut Microbiota on Cardio-Metabolic Parameters and Immunity in Coronary Artery Disease Patients with and without Type-2 Diabetes Mellitus. Front. Microbiol. 2017, 8, 1936. [Google Scholar] [CrossRef]
- Wu, W.K.; Panyod, S.; Ho, C.T.; Kuo, C.H.; Wu, M.S.; Sheen, L.Y. Dietary Allicin Reduces Transformation of L-Carnitine to TMAO through Impact on Gut Microbiota. J. Funct. Foods 2015, 15, 408–417. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Utrera, M.; Morcuende, D.; Rodríguez-Carpena, J.G.; Estévez, M. Fluorescent HPLC for the Detection of Specific Protein Oxidation Carbonyls—α-Aminoadipic and γ-Glutamic Semialdehydes—In Meat Systems. Meat Sci. 2011, 89, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, P.; Yang, X.; Chen, M.; Dong, Y.; Li, J. Untargeted Metabolomics Unravel Serum Metabolic Alterations in Smokers with Hypertension. Front. Physiol. 2023, 14, 1127294. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut Microbiome, Obesity, and Metabolic Dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Garcia-mantrana, I.; Selma-royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.W.; Lee, C.L.; Liu, W.J. Lower All-Cause Mortality for Coronary Heart or Stroke Patients Who Adhere Better to Mediterranean Diet-An. Nutrients 2022, 14, 3203. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salvadó, J.S.; Covas, M.; Corella, D.; Arós, F.; Gracia, E.G.; Gutiérrez, V.R.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A Carvacrol-Thymol Blend Decreased Intestinal Oxidative Stress and Influenced Selected Microbes without Changing the Messenger RNA Levels of Tight Junction Proteins in Jejunal Mucosa of Weaning Piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Scherer, R.; Junior, S.B.; de Albuquerque, R.; Godoy, H.T. Microencapsulated Eucalyptol and Eugenol as Growth Promoters in Broilers. Braz. J. Food Res. 2014, 5, 26–32. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Fan, G.; Ren, J.N.; Zhang, L.L.; Pan, S.Y. Effects of Orange Essential Oil on Intestinal Microflora in Mice. J. Sci. Food Agric. 2019, 99, 4019–4028. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yu, X.F.; Kerem, G.; Ren, P.G. Perturbation on Gut Microbiota Impedes the Onset of Obesity in High Fat Diet-Induced Mice. Front. Endocrinol. 2022, 13, 795371. [Google Scholar] [CrossRef] [PubMed]
- Zununi Vahed, S.; Barzegari, A.; Zuluaga, M.; Letourneur, D.; Pavon-Djavid, G. Myocardial Infarction and Gut Microbiota: An Incidental Connection. Pharmacol. Res. 2018, 129, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lv, L.; Liu, B.; Wang, S.; Zhang, S.; Wu, Z.; Yang, L.; Bian, X.; Wang, Q.; Wang, K.; et al. Akkermansia Muciniphila Ameliorates Acetaminophen-Induced Liver Injury by Regulating Gut Microbial Composition and Metabolism. Microbiol. Spectr. 2022, 10, e01596-21. [Google Scholar] [CrossRef] [PubMed]
- Hotz, P.W.; Müller, S.; Mendler, L. SUMO-Speci Fi c Isopeptidases Tuning Cardiac SUMOylation in Health and Disease. Front. Mol. Biosci. 2021, 8, 786136. [Google Scholar] [CrossRef]
- Pober, J.; Min, W. SRF SUMOylation Modulates Smooth Muscle Phenotypic Switch and Vascular Remodeling; Research Square: Durham, NC, USA, 2023; ISBN 0000000224796. [Google Scholar]
- Xiu, D.; Wang, Z.; Cui, L.; Jiang, J.; Yang, H.; Liu, G. Sumoylation of SMAD 4 Ameliorates the Oxidative Stress-Induced Apoptosis in Osteoblasts. Cytokine 2018, 102, 173–180. [Google Scholar] [CrossRef]
- Stankovic-valentin, N.; Melchior, F. Molecular Aspects of Medicine Control of SUMO and Ubiquitin by ROS: Signaling and Disease Implications. Mol. Asp. Med. 2018, 63, 3–17. [Google Scholar] [CrossRef]
- Savarese, M.; Sarparanta, J.; Vihola, A. Panorama of the Distal Myopathies. Acta Myol. 2020, 39, 245–265. [Google Scholar] [CrossRef]
- Pavadai, E.; Rynkiewicz, M.J.; Yang, Z.; Gould, I.R. Modulation of Cardiac Thin Filament Structure by Phosphorylated Troponin-I Analyzed by Protein-Protein Docking and Molecular Dynamics Simulation. Arch. Biochem. Biophys. 2022, 725, 109282. [Google Scholar] [CrossRef]
- Chalovich, J.M.; Zhu, L.; Johnson, D.; Irving, T.C. Hypertrophic Cardiomyopathy Mutations of Troponin Reveal Details of Striated Muscle Regulation. Front. Physiol. 2022, 13, 902079. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; Reis Lívero, F.A.D.; de Souza, P.; de Almeida, D.A.T.; Donadel, G.; Lourenço, E.L.B.; Gasparotto, A., Jr. Natural Products as Modulators of Mitochondrial Dysfunctions Associated with Cardiovascular Diseases: Advances and Opportunities. J. Med. Food 2023, 26, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, B.; Zhou, Z. Preclinical Studies of Natural Products Targeting the Gut Microbiota: Beneficial Effects on Diabetes. J. Agric. Food Chem. 2022, 70, 8569–8581. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.T.; Cao, D.; Lin, C.; Chung, S.K.; Chung, S.S. Tissue-Specific Expression of Two Aldose Reductase-like Genes in Mice: Abundant Expression of Mouse Vas Deferens Protein and Fibroblast Growth Factor-Regulated Protein in the Adrenal Gland. Biochem. J. 1995, 312, 609–615. [Google Scholar] [CrossRef]
- Penning, T.M.; Palackal, N.T.; Lee, S.H.; Blair, I.; Yu, D.; Berlin, J.A.; Field, J.M.; Harvey, R.G. Aldo-Keto Reductases and the Metabolic Activation of Polycyclic Aromatic Hydrocarbons. ACS Symp. Ser. 2003, 865, 83–99. [Google Scholar] [CrossRef]
- Bogner, A.N.; Stiers, K.M.; Tanner, J.J. Structure, Biochemistry, and Gene Expression Patterns of the Proline Biosynthetic Enzyme Pyrroline-5-Carboxylate Reductase (PYCR), an Emerging Cancer Therapy Target. Amino Acids 2021, 53, 1817–1834. [Google Scholar] [CrossRef]
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein Carbonylation in Food and Nutrition: A Concise Update. Amino Acids 2022, 54, 559–573. [Google Scholar] [CrossRef]
- Hecker, M.; Wagner, A.H. Role of Protein Carbonylation in Diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Bollineni, R.C.; Fedorova, M.; Blüher, M.; Hoffmann, R. Carbonylated Plasma Proteins as Potential Biomarkers of Obesity Induced Type 2 Diabetes Mellitus. J. Proteome Res. 2014, 13, 5081–5093. [Google Scholar] [CrossRef]
- Almogbel, E.; Rasheed, N. Elevated Levels of Protein Carbonylation in Patients with Diabetic Nephropathy: Therapeutic and Diagnostic Prospects. Am. J. Med. Sci. 2019, 358, 26–32. [Google Scholar] [CrossRef]
- Xu, C.C.; Yang, S.F.; Zhu, L.H.; Cai, X.; Sheng, Y.S.; Zhu, S.W.; Xu, J.X. Regulation of N-Acetyl Cysteine on Gut Redox Status and Major Microbiota in Weaned Piglets. J. Anim. Sci. 2014, 92, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, X.L.; Le, G.W.; Shi, Y.H. Lactobacilli Prevent Hydroxy Radical Production and Inhibit Escherichia Coli and Enterococcus Growth in System Mimicking Colon Fermentation. Lett. Appl. Microbiol. 2010, 50, 264–269. [Google Scholar] [CrossRef] [PubMed]






| Protein Name | Gene Name | p-Value | Fold-Change | Biological Function | Accession Number | Treatment/Dose |
|---|---|---|---|---|---|---|
| Aldose reductase-related protein 2 | AKR1B8 | 0.03 | −0.98 | Carbonyl metabolism | P45377 | Thyme/10 |
| Isoform 2 of Aspartyl/asparaginyl beta-hydroxylase | ASPH | <0.0001 <0.0001 | −0.99 −1.11 | Cardiac contraction | Q8BSY0 | Thyme/10 Thyme/20 |
| Tropomyosin beta chain | TPM2 | 0.01 0.006 | −0.79 −0.87 | Cardiac contraction | P58774 | Thyme/10 Thyme/20 |
| Isoform 5 of LIM domain-binding protein 3 | LDB3 | <0.0001 | −1.81 | Cardiac contraction | Q9JKS4 | Oregano/10 |
| Small ubiquitin-related modifier 3 | SUMO3 | 0.003 0.002 | −1.49 −1.64 | SUMOylation, process regulated by cellular oxidative stress | Q9Z172 | Oregano/10 Oregano/20 |
| NADH-ubiquinone oxidoreductase chain 4 | MTND4 | 0.04 | −1.34 | Mitochondrial respiratory chain | P03911 | Oregano/20 |
| Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial | ALDH4A1 | 0.03 | −0.89 | Carbonyl metabolism | Q8CHT0 | Oregano/10 |
| Alcohol dehydrogenase [NADP (+)] | AKR1A1 | 0.03 | −0.61 | Carbonyl metabolism | Q9JII6 | Oregano/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Quintero, M.J.; Delgado, J.; Martín Chaves, L.; Medina-Vera, D.; Murri, M.; Becerra-Muñoz, V.M.; Estévez, M.; Crespo-Leiro, M.G.; Paz López, G.; González-Jiménez, A.; et al. Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice. Antioxidants 2023, 12, 1643. https://doi.org/10.3390/antiox12081643
Sánchez-Quintero MJ, Delgado J, Martín Chaves L, Medina-Vera D, Murri M, Becerra-Muñoz VM, Estévez M, Crespo-Leiro MG, Paz López G, González-Jiménez A, et al. Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice. Antioxidants. 2023; 12(8):1643. https://doi.org/10.3390/antiox12081643
Chicago/Turabian StyleSánchez-Quintero, María José, Josué Delgado, Laura Martín Chaves, Dina Medina-Vera, Mora Murri, Víctor M. Becerra-Muñoz, Mario Estévez, María G. Crespo-Leiro, Guillermo Paz López, Andrés González-Jiménez, and et al. 2023. "Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice" Antioxidants 12, no. 8: 1643. https://doi.org/10.3390/antiox12081643
APA StyleSánchez-Quintero, M. J., Delgado, J., Martín Chaves, L., Medina-Vera, D., Murri, M., Becerra-Muñoz, V. M., Estévez, M., Crespo-Leiro, M. G., Paz López, G., González-Jiménez, A., A. G. Ranea, J., Queipo-Ortuño, M. I., Plaza-Andrades, I., Rodríguez-Capitán, J., Pavón-Morón, F. J., & Jiménez-Navarro, M. F. (2023). Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice. Antioxidants, 12(8), 1643. https://doi.org/10.3390/antiox12081643