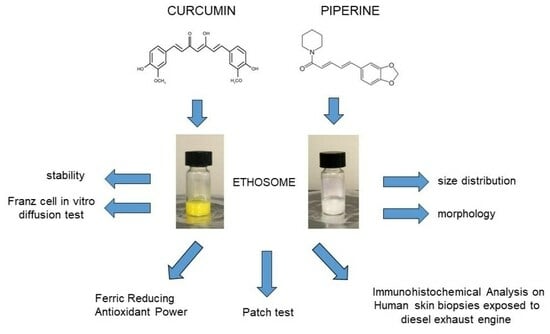
Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ethosomes
2.2.1. Bulk Approach
2.2.2. Microfluidic Approach
2.3. Transmission Electron Microscopy (TEM)
2.4. Photon Correlation Spectroscopy (PCS)
2.5. Assessment of Entrapment Capacity
2.6. Franz Cell Methodology
2.7. In Vitro Release Tests (IVRTs)
2.8. In Vitro Permeation Tests (IVPTs)
2.9. HPLC Analysis
2.10. Ferric Reducing Antioxidant Power (FRAP Test)
2.11. Patch Test
2.12. Biological Activity Studies
2.12.1. Human Specimens
2.12.2. Treatment with Formulations and DEEExposure
2.12.3. Tissue Collection and Immunohistochemical Analysis
2.13. Statistical Analysis
3. Results
3.1. Preparation of Ethosomes
3.2. Drug Loading in Ethosomes
Drug Entrapment Capacity
3.3. Evaluation of Antioxidant Activity
3.4. IVRT
3.5. IVPT
3.6. In Vivo Comparative Irritation Test
3.7. Biological Activity Studies
Immunohistochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.O.; Uchida, Y.; Park, K. Diesel Particulate Extract Accelerates Premature Skin Aging in Human Fibroblasts via Ceramide-1-Phosphate-Mediated Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2691. [Google Scholar] [CrossRef]
- Valacchi, G.; Pambianchi, E.; Coco, S.; Pulliero, A.; Izzotti, A. MicroRNA Alterations Induced in Human Skin by Diesel Fumes, Ozone, and UV Radiation. J. Pers. Med. 2022, 12, 176. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Özbey Yücel, Ü.; Altiner, S.; Ozdel Oztürk, B.; Cerci, P.; Türk, M.; Gorgülü Akin, B.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The External Exposome and Allergies: From the Perspective of the Epithelial Barrier Hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Yadav, S.S.; Singh, M.K.; Hussain, S.; Dwivedi, P.; Khattri, S.; Singh, K. Therapeutic spectrum of piperine for clinical practice: A scoping review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5813–5840. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2181. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phyther. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, N.; Asoodeh, A. Co-administration of curcumin with other phytochemicals improves anticancer activity by regulating multiple molecular targets. Phytother. Res. 2023, 37, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Peng, W.; Hu, H.; Cao, T.; Hou, G. Effect of the Single and Combined Use of Curcumin and Piperine on Growth Performance, Intestinal Barrier Function, and Antioxidant Capacity of Weaned Wuzhishan Piglets. Front. Vet. Sci. 2020, 7, 418. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 8, 735–748. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phyther. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef]
- Lu, H.; Gong, H.; Du, J.; Gao, W.; Xu, J.; Cai, X.; Yang, Y.; Xiao, H. Piperine ameliorates psoriatic skin inflammation by inhibiting the phosphorylation of STAT3. Int. Immunopharmacol. 2023, 119, 110221. [Google Scholar] [CrossRef] [PubMed]
- Jantarat, C.; Sirathanarun, P.; Boonmee, S.; Meechoosin, W.; Wangpittaya, H. Effect of piperine on skin permeation of curcumin from a bacterially derived cellulose-composite double-layer membrane for transdermal curcumin delivery. Sci. Pharm. 2018, 86, 39. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Safavi, M.S.; Handali, S.; Nosrati, M.; Shojaosadati, S.A. Synergistic Effect of Self-assembled Curcumin- Piperine-Loaded Human Serum Albumin Nanoparticles on Suppressing Cancer Cells. Drug Dev. Ind. Pharm. 2020, 10, 1647–1655. [Google Scholar] [CrossRef]
- Ahmad, S.; Hafeez, A. Formulation and Development of Curcumin—Piperine—Loaded S—SNEDDS for the Treatment of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Mariani, P.; Contado, C.; Drechsler, M.; Puglia, C.; Cortesi, R. Curcumin containing monoolein aqueous dispersions: A preformulative study. Mater. Sci. Eng. C 2013, 33, 4923–4934. [Google Scholar] [CrossRef]
- Politi, F.A.S.; Carvalho, S.G.; Rodero, C.F.; dos Santos, K.P.; Meneguin, A.B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. Piperine-loaded nanoparticles incorporated into hyaluronic acid/sodium alginate-based membranes for the treatment of inflammatory skin diseases. Int. J. Biol. Macromol. 2023, 227, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Sahoo, P.K.; Manchanda, S. Curcumin Loaded Ethosomal Gel for Improved Topical Delivery: Formulation, Characterization and Ex-vivo Studies. Pharm. Nanotechnol. 2021, 9, 281–287. [Google Scholar] [CrossRef]
- Upadhyay, P.; Singh, D.; Upadhyay, S. Vesicular Approach Review on Nanocarriers bearing Curcumin and Applications. Recent Adv. Drug Deliv. Formul. 2022, 16, 256–269. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.; Kumbar, V.; Patil, S.; Joshi, S.; Bhat, K.; Diwan, P. Factorial design based curcumin ethosomal nanocarriers for the skin cancer delivery: In vitro evaluation. J. Liposome Res. 2019, 29, 291–311. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D.K.; Ashawat, M.S. Topical creams of piperine loaded lipid nanocarriers for management of atopic dermatitis: Development, characterization, and in vivo investigation using BALB/c mice model. J. Liposome Res. 2022, 32, 62–73. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Dalla Pozza, E.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. Int. J. Mol. Sci. 2022, 23, 15112. [Google Scholar] [CrossRef] [PubMed]
- Hyeon TI, Y.K. Ethosome Containing Ceramide as a Skin Carrier of Active Ingredients. Curr. Drug Deliv. 2023, 20, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Pozos-Nonato, S.; Domínguez-Delgado, C.L.; Campos-Santander, K.A.; Benavides, A.A.; Pacheco-Ortin, S.M.; Higuera-Piedrahita, R.I.; Resendiz-González, G.; Molina-Trinidad, E.M. Novel Nanotechnological Strategies for Skin Anti-aging. Curr. Pharm. Biotechnol. 2023, 24, 1397–1419. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. aQbD as a platform for IVRT method development—A regulatory oriented approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef]
- Ferrara, F.; Benedusi, M.; Cervellati, F.; Sguizzato, M.; Montesi, L.; Bondi, A.; Drechsler, M.; Pula, W.; Valacchi, G.; Esposito, E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. Int. J. Mol. Sci. 2022, 23, 8756. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Draft Guideline on Quality and Equivalence of Topical Products; Ema/Chmp/Qwp/708282/2018; European Medicines Agency: Amsterdam, The Netherlands, 2018; Volume 44, pp. 1–36. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.P.; Choudhary, H.; Valacchi, G. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.S.; Jung, H.S. Precise control of liposome size using characteristic time depends on solvent type and membrane properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef]
- Syed, S.B.; Arya, H.; Fu, I.H.; Yeh, T.K.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Patial, V.; Mahesh, S.; Sharma, S.; Pratap, K.; Singh, D.; Padwad, Y.S. Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2015, 40, 445–452. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Liu, J.; He, Z.; Ding, C.; Huang, G.; Zhou, W.; Zhou, L. Preparation of a ligustrazine ethosome patch and its evaluation in vitro and in vivo. Int. J. Nanomed. 2011, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeial Convention, I.R. 〈1724〉 Semisolid Drug Products—Performance Tests. Usp 2014, 37, 1273–1284. [Google Scholar]
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using franz diffusion cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using Spss; SAGE Publications Inc.: London, UK, 2009; ISBN 9781847879066. [Google Scholar]
- Lin, B.; Wang, W.; Ba, W.; Li, H.; Fan, J. Preparation and partial pharmacodynamic studies of luliconazole ethosomes. Clin. Exp. Pharmacol. Physiol. 2022, 49, 549–557. [Google Scholar] [CrossRef]
- Aljohani, A.A.; Alanazi, M.A.; Munahhi, L.A.; Hamroon, J.D.; Mortagi, Y.; Qushawy, M.; Soliman, G.M. Binary ethosomes for the enhanced topical delivery and antifungal efficacy of ketoconazole. OpenNano 2023, 11, 100145. [Google Scholar] [CrossRef]
- Abd El-Alim, S.H.; Kassem, A.A.; Basha, M.; Salama, A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 563, 293–303. [Google Scholar] [CrossRef]
- Raghuvanshi, A.; Shah, K.; Dewangan, H.K. Ethosome as antigen delivery carrier: Optimisation, evaluation and induction of immunological response via nasal route against hepatitis B. J. Microencapsul. 2022, 39, 352–363. [Google Scholar] [CrossRef]
- Pilch, E.; Musiał, W. Liposomes with an ethanol fraction as an application for drug delivery. Int. J. Mol. Sci. 2018, 19, 3806. [Google Scholar] [CrossRef]
- El-Shenawy, A.A.; Abdelhafez, W.A.; Ismail, A.; Kassem, A.A. Formulation and Characterization of Nanosized Ethosomal Formulations of Antigout Model Drug (Febuxostat) Prepared by Cold Method: In Vitro/Ex Vivo and In Vivo Assessment. AAPS PharmSciTech 2020, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. BioFactors 2019, 45, 536–547. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Gupta, R.L. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Environmental Air Pollutants Affecting Skin Functions with Systemic Implications. Int. J. Mol. Sci. 2023, 24, 10502. [Google Scholar] [CrossRef]
- Prieux, R.; Eeman, M.; Rothen-Rutishauser, B.; Valacchi, G. Mimicking cigarette smoke exposure to assess cutaneous toxicity. Toxicol. Vitr. 2020, 62, 104664. [Google Scholar] [CrossRef]
- Hieda, D.S.; Anastacio da Costa Carvalho, L.; Vaz de Mello, B.; de Oliveira, E.A.; Romano de Assis, S.; Wu, J.; Du-Thumm, L.; Viana da Silva, C.L.; Roubicek, D.A.; Maria-Engler, S.S.; et al. Air Particulate Matter Induces Skin Barrier Dysfunction and Water Transport Alteration on a Reconstructed Human Epidermis Model. J. Investig. Dermatol. 2020, 140, 2343–2352.e3. [Google Scholar] [CrossRef]
- Ivarsson, J.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Blueberry Supplementation and Skin Health. Antioxidants 2023, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Pambianchi, E.; Ferrara, F.; Pecorelli, A.; Woodby, B.; Grace, M.; Therrien, J.P.; Lila, M.A.; Valacchi, G. Blueberry Extracts as a Novel Approach to Prevent Ozone-Induced Cutaneous Inflammasome Activation. Oxid. Med. Cell. Longev. 2020, 2020, 9571490. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, X.; Zhang, X.; Cao, W.; Wang, Y.; Chen, H.; Wang, T.; Tsai, H.I.; Zhang, R.; Chang, D.; et al. The effects of quercetin-loaded PLGA-TPGS nanoparticles on ultraviolet B-induced skin damages in vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phyther. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferulolylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Huang, J.; Yi, Q.; You, Y.; Chen, Y.; Niu, T.; Li, Y.; Zhang, J.; Ji, X.; Xu, G.; Zou, W.; et al. Curcumin suppresses oxidative stress via regulation of ROS/NF-κB signaling pathway to protect retinal vascular endothelial cell in diabetic retinopathy. Mol. Cell. Toxicol. 2021, 17, 367–376. [Google Scholar] [CrossRef]
- Pradeep, C.R.; Kuttan, G. Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int. Immunopharmacol. 2004, 4, 1795–1803. [Google Scholar] [CrossRef]
- Saha, P.; Durugkar, S.; Jain, S.; Shantanu, P.A.; Panda, S.R.; Jala, A.; Gokhale, S.; Sharma, P.; Naidu, V.G.M. Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis. Int. J. Mol. Sci. 2022, 23, 14722. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in Photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef] [PubMed]




| TFR a (μL/min) | FRR b | FAP c (μL/min) | FLP d (μL/min) | PC e (%, w/w) | Ethanol (%, w/w) | Water (%, w/w) | Z Average f ± s.d. (nm) | D.I. g ± s.d. |
|---|---|---|---|---|---|---|---|---|
| 12 | 2:1 | 4 + 4 | 4 | 0.9 | 29.1 | 70 | 192.30 ± 25.33 | 0.129 ± 0.013 |
| 24 | 2:1 | 6 + 6 | 6 | 0.9 | 29.1 | 70 | 236.40 ± 47.65 | 0.203 ± 0.032 |
| 36 | 2:1 | 12 + 12 | 12 | 0.9 | 29.1 | 70 | 296.17 ± 20.76 | 0.379 ± 0.013 |
| 60 | 2:1 | 20 + 20 | 20 | 0.9 | 29.1 | 70 | 308.01 ± 60.85 | 0.285 ± 0.119 |
| 90 | 2:1 | 30 + 30 | 30 | 0.9 | 29.1 | 70 | 282.61 ± 27.42 | 0.231 ± 0.048 |
| Formulation | PC 1 (%, w/w) | Ethanol (%, w/w) | Water (%, w/w) | PIP 2 (%, w/w) | CUR 3 (%, w/w) |
|---|---|---|---|---|---|
| ET | 0.9 | 29.100 | 70 | - | - |
| ET–PIP | 0.9 | 29.075 | 70 | 0.025 | - |
| ET–CUR | 0.9 | 29.075 | 70 | - | 0.025 |
| Formulation | Time (Days) | Z-Average (nm) ± s.d. | Dispersity Index ± s.d. |
|---|---|---|---|
| ET | 1 | 206.32 ± 33.22 | 0.144 ± 0.012 |
| 60 | 230.36 ± 23.11 | 0.155 ± 0.032 | |
| ET–PIP | 1 | 213.13 ± 13.18 | 0.129 ± 0.019 |
| 60 | 269.8 ± 15.13 | 0.196 ± 0.069 | |
| ET–CUR | 1 | 213.03 ± 8.25 | 0.127 ± 0.046 |
| 60 | 242.85 ± 9.97 | 0.189 ± 0.00 |
| Formulation | EC 1 % |
|---|---|
| ET–PIP | 78.95 ± 6.47 |
| ET–CUR | 96.87 ± 4.31 |
| Formulation | FRAP 1 (µmol TE/g) |
|---|---|
| ET | n.a. 1 |
| SOL–PIP | n.a. 1 |
| ET–PIP | n.a. 1 |
| SOL–CUR | 3042.86 ± 191.73 |
| ET–CUR | 3097.27 ± 112.83 * |
| SOL–PIP + SOL–CUR | 2996.50 ± 95.54 |
| ET–PIP + ET–CUR | 3408.87 ± 68.80 * |
| Formulation | R 1 (µg/cm2/h) | A 2 (µg/cm2) | Zero-Order Plot (R2) | First-Order Plot (R2) | Higuchi Plot (R2) | Peppas Plot (n/R2) |
|---|---|---|---|---|---|---|
| ET–CUR | 15.961 ± 2.52 | 43.87 ± 8.28 | 0.935 | 0.947 | 0.996 | 0.54/0.995 |
| SOL–CUR | 25.67 ± 5.41 | 67.75 ± 5.50 | - | - | - | - |
| ET–PIP | 27.88 ± 6.04 | 76.65 ± 7.52 | 0.805 | 0.843 | 0.967 | 0.43/0.954 |
| SOL–PIP | 52.08 ± 4.24 | 148.49 ± 10.45 | - | - | - | - |
| IVPT Parameters | ET–CUR | SOL–CUR | ET–PIP | SOL–PIP |
|---|---|---|---|---|
| Jss 1 (μg/cm2/h) | 0.70 ± 0.21 | 0.32 ± 0.1 | 5.27 ± 2.2 | 8.85 ± 3.1 |
| Kp 2 (cm/h) × 103 | 3.12 ± 0.94 | 1.28 ± 0.40 | 23.11 ± 9.65 | 43.81 ± 15.35 |
| Tlag 3 (h) | 5.03 ± 0.81 | 4.74 ± 0.33 | 1.06 ± 0.052 | 1.84 ± 0.23 |
| D 4 (cm2 h−1) × 105 | 3.33 ± 0.54 | 3.53 ± 0.25 | 15.80 ± 0.76 | 9.10 ± 1.14 |
| P 5 membrane/vehicle | 2.97 ± 1.37 | 1.15 ± 0.44 | 4.64 ± 2.16 | 15.26 ± 7.25 |
| A 6 (µg/cm−2) | 24.67 ± 4.1 | 3.92 ± 0.42 | 38.40 ± 5.67 | 48.66 ± 7.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, F.; Bondi, A.; Pula, W.; Contado, C.; Baldisserotto, A.; Manfredini, S.; Boldrini, P.; Sguizzato, M.; Montesi, L.; Benedusi, M.; et al. Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage. Antioxidants 2024, 13, 91. https://doi.org/10.3390/antiox13010091
Ferrara F, Bondi A, Pula W, Contado C, Baldisserotto A, Manfredini S, Boldrini P, Sguizzato M, Montesi L, Benedusi M, et al. Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage. Antioxidants. 2024; 13(1):91. https://doi.org/10.3390/antiox13010091
Chicago/Turabian StyleFerrara, Francesca, Agnese Bondi, Walter Pula, Catia Contado, Anna Baldisserotto, Stefano Manfredini, Paola Boldrini, Maddalena Sguizzato, Leda Montesi, Mascia Benedusi, and et al. 2024. "Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage" Antioxidants 13, no. 1: 91. https://doi.org/10.3390/antiox13010091
APA StyleFerrara, F., Bondi, A., Pula, W., Contado, C., Baldisserotto, A., Manfredini, S., Boldrini, P., Sguizzato, M., Montesi, L., Benedusi, M., Valacchi, G., & Esposito, E. (2024). Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage. Antioxidants, 13(1), 91. https://doi.org/10.3390/antiox13010091