Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plant Material
2.3. Preparation of Plant Extract
2.4. Chemicals
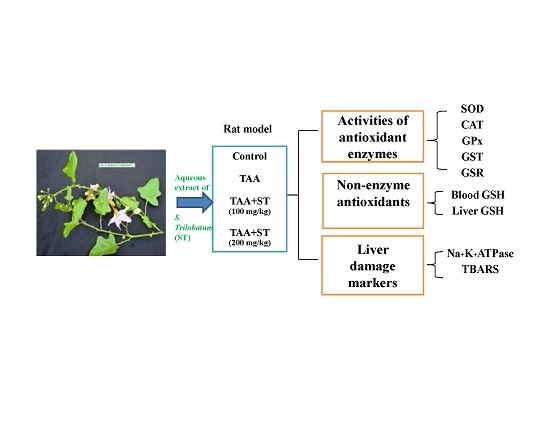
2.5. Experimental Design
2.6. Assay of Antioxidant Enzymes
2.6.1. Reduced Glutathione (GSH) Assay
2.6.2. Superoxide Dismutase (SOD) Assay
2.6.3. Catalase Activity (CAT)
2.6.4. Glutathione Peroxidase (GPx) Assay
2.6.5. Glutathione-S-Transferase (GST) Assay
2.6.6. Glutathione Reductase (GSR) Assay
2.6.7. Estimation of Lipid Peroxidation (TBARS) Assay
2.6.8. Measurement of Na+ K+ ATPase Activity
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| ATP | adenosine triphosphate |
| b.w | body weight |
| CAT | catalase |
| CDNB | 1 chloro 2, 4 dinitrobenzene |
| cm | centimeter |
| DNA | deoxy ribonucleic acid |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DTNB | 5,5-dithiobis-2-nitrobenzoic acid |
| EDTA | ethylene diamine tetra acetic acid |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GST | glutathione-S-transferase |
| HO• | hydroxyl radicals |
| H2O2 | hydrogen peroxide |
| Kg | kilogram |
| MDA | melondialdehyde |
| mg | milligram |
| MgCl2 | magnesium chloride |
| mM | millimolar |
| Na+-K+-ATPase | sodium-potassium adenosine triphosphatase |
| NADH | nicotinamide adenine dinucleotide hydrogen |
| NADPH | nicotinamide adenine dinucleotide hydrogen phosphate; nm-nanometer |
| O2• | oxygen radicals |
| °C | degree Celsius |
| p.o. | per oral |
| ROS | reactive oxygen species |
| s.c. | subcutaneous |
| S.D. | standard deviation |
| SOD | superoxide dismutase |
| TAA | thioacetamide |
| TBA- | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
| TCA | trichloroacetic acid |
| μM | micro molar |
References
- Gutteridge, J.M.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Common. 2010, 393, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Lipid peroxidation oxygen radicals, cell damage, and antioxidant therapy. Lancet 1994, 1, 1396–1397. [Google Scholar] [CrossRef]
- Scholz, R.W.; Reddy, P.V.; Wynn, M.K.; Graham, K.S.; Liken, A.D.; Gumpricht, E.; Reddy, C.C. Glutathione-dependent factors, and inhibition of rat liver microsomal lipid peroxidation. Free Radic. Biol. Med. 1997, 23, 815–828. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Pristijono, P.; Golding, J.B.; Bowyer, M.C.; Scarlett, C.J.; Stathopoulos, C.E. Enhancing the total phenolic content and antioxidants of lemon pomace aqueous extracts by applying UV-C irradiation to the dried powder. Foods 2016, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in the Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Lee, T.H. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Liu, A.; Anadón, A.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; Yuan, Z.; Martínez, M.A. Paracetamol: Overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab. Rev. 2017, 2, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.R.; Dilworth, L.L.; Thompson, R.K.; Alexander-Lindo, R.L.; Omoruyi, F.O. Effects of combined inositol hexakisphosphate and inositol supplement on antioxidant activity and metabolic enzymes in the liver of streptozotocin-induced type 2 diabetic rats. Chem. Biol. Interact. 2017, 275, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, B.; Shi, W.; Zuo, H.; Cui, D.; Ni, L.; Chen, J. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol. 2015, 36, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rat. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Rodrigues, B.P.; Campagnaro, B.P.; Balarini, C.M.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil ameliorates biomarkers of genotoxicity in an experimental model of spontaneous atherosclerosis. Lipids Health Dis. 2013, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Patel, P.R.; Kajal, S.S. Antioxidant activity of some selected medicinal plants in western region of India. Adv. Biol. Res. 2010, 4, 23–26. [Google Scholar]
- Li, X.; Benjamin, I.S.; Alexander, B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J. Hepatol. 2002, 36, 488–493. [Google Scholar] [CrossRef]
- Okuyama, H.; Makamura, H.; Shimahara, Y.; Uyama, N.; Kwon, Y.W.; Yamaoka, N.; Yodoi, J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J. Hepatol. 2002, 42, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-N.; Maitra, A.; Lee, K.-F.; Jan, Y.-Y.; Chen, M.-F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis 2004, 25, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, K.M. The Indian Materia Medica, 3rd ed.; Popular Book Depot: Bombay, India, 1976; Volume 1, pp. 1153–1154. [Google Scholar]
- Kiritikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; Bishen Singh Mahendra Pal Singh: Dehradun, India, 1999; Volume 3, p. 1762. [Google Scholar]
- Govindan, S.; Viswanathan, S.; Vijayasekaran, V.; Alagappan, R. A pilot study on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. J. Ethnopharmacol. 1999, 66, 205–210. [Google Scholar] [CrossRef]
- Govindan, S.; Viswanathan, S.; Vijayasekaran, V.; Alagappan, R. Further studies on the clinical efficacy of Solanum trilobatum in bronchial asthma. Phytother. Res. 2004, 18, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Sastri, B.N. The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products; CSIR: New Delhi, India, 1972; Volume 6, pp. 395–396. [Google Scholar]
- Pandurangan, A.; Lal Khosa, R.; Hemalatha, S. Evaluation of analgesic potential of Solanum trilobatum roots. Iran. J. Pharm. Res. 2009, 8, 269–273. [Google Scholar]
- Mohanan, P.V.; Rao, J.M.; Kutty, M.A.S.; Devi, K.S. Cytotoxicity and anti-carcinogenic activity of Sobatum. Biomedicine 1998, 18, 106–111. [Google Scholar]
- Mohan, P.V.; Devi, K.S. Cytotoxic potential of the preparations from Solanum trilobatum and the effect of Sobatum on tumor reduction in mice. Cancer Lett. 1996, 110, 71–76. [Google Scholar] [CrossRef]
- Mohanan, P.V.; Devi, K.S. Toxicological evaluation of sobatum. Cancer Lett. 1998, 127, 135–140. [Google Scholar] [CrossRef]
- Shahjahan, M.; Sabitha, K.E.; Mallika Devi, R.; Shyamala, C.S. Effect of medicinal plants on tumourigenesis. Indian J. Med. Res. 2004, 123, 23–27. [Google Scholar]
- Shahjahan, M.; Vani, G.; Shyamaladevi, C.S. Effect of Solanum trilobatum on the antioxidant status during diethyl nitrosamine-induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem. Biol. Interact. 2005, 156, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Latha, P.S.; Kannabiran, K. Antimicrobial activity, and phytochemicals of Solanum trilobatum Linn. Afr. J. Biotechnol. 2006, 5, 2402–2404. [Google Scholar]
- Jahan, M.S.; Vani, G.; Shyamaladevi, C.S. Effect of Solanum trilobatum on hepatic drug metabolizing enzymes during diethylnitrosamine-induced hepatocarcinogenesis promoted by phenobarbital in rat. Hepatol. Res. 2007, 37, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.N.; Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Combination therapeutic effect of cisplatin along with Solanum trilobatum on benzo(a)pyrene induced experimental lung carcinogenesis. Nat. Prod. Res. 2008, 22, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.M.; Kandasamy, S.; Chinnappa, R. Comparative antimicrobial activity of callus and natural plant extracts of Solanum trilobatum L. Anc. Sci. Life 2009, 28, 3–5. [Google Scholar] [PubMed]
- Govindarajan, P.; Chinnachamy, C. Phytochemical and therapeutic evaluation of leaf and in vitro derived callus and shoot of Solanum trilobatum. Pak. J. Pharm. Sci. 2014, 27, 2101–2107. [Google Scholar] [PubMed]
- Ramar, M.; Manikandan, B.; Marimuthu, P.N.; Raman, T.; Mahalingam, A.; Subramanian, P.; Karthick, S.; Munusamy, A. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Sini, H.; Devi, K.S. Antioxidant activities of the chloroform extract of Solanum trilobatum. Pharm. Biol. 2004, 42, 462–466. [Google Scholar] [CrossRef]
- Ahmed, K.Z.; Sidhra, S.Z.; Ponmurugan, P.; Kumar, B.S. Ameliorative potential of Solanum trilobatum leaf extract and fractions on lipid profile and oxidative stress in experimental diabetes. Pak. J. Pharm. Sci. 2016, 29, 1578. [Google Scholar] [PubMed]
- Pandurangan, A.; Khosa, R.L.; Hemalatha, S. Antinociceptive activity of steroid alkaloids isolated from Solanum trilobatum Linn. J. Asian Nat. Prod. Res. 2010, 12, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, S. Possible Industrial application of genus Solanum in twenty-first century—A review. J. Sci. Ind. Res. 2004, 63, 116–124. [Google Scholar]
- Emmanuel, S.; Ignacimuthu, S.; Perumalsamy, R.; Amalraj, T. Anti-inflammatory activity of Solanum trilobatum. Fitoterapia 2006, 77, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.; Lal Khosa, R.; Hemalatha, S. Evaluation of anti-inflammatory and analgesic activity of root extract of Solanum Trilobatum Linn. Iran. J. Pharm. Res. 2008, 7, 217–221. [Google Scholar]
- Pandurangan, A.; Khosa, R.L.; Hemalatha, S. Anti-inflammatory activity of an alkaloid from Solanum trilobatum on acute and chronic inflammation models. Nat. Prod. Res. 2011, 25, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, S.; Elumalai, K.; Jeyasankar, A. Mosquitocidal properties of Solanum trilobatum L. (Solanaceae) leaf extracts against three important human vector mosquitoes (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2013, 6, 854–858. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S.; Kamaraj, C.; Bagavan, A.; Zahir, A.A.; Kirthi, A.V.; Elango, G.; et al. Solanum trilobatum extract-mediated synthesis of titanium dioxide nanoparticles to control Pediculus humanus capitis, Hyalomma anatolicum anatolicum and Anopheles subpictus. Parasitol. Res. 2014, 113, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.; Amalan Rabert, G.; Jeyaseelan, D. Rapid proliferation of multiple shoots in Solanum trilobatum L. Plant Tissue Cult. 2004, 14, 107–112. [Google Scholar]
- Vijaimohan, K.; Mallika, J.; Shyamala, D.C. Chemoprotective effect of Sobatum against lithium-induced oxidative damage in rats. J. Young Pharm. 2010, 2, 68–73. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Kumar, G.; Sharmila Banu, G.; Vanitha Pappa, P.; Sundararajan, M.; Rajasekara Pandian, M. Hepatoprotective activity of Trianthema portulacastrum L against paracetamol and thioacetamide intoxication in albino rats. J. Ethnopharmacol. 2004, 92, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of blood protein-bound sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–197. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of SOD. Indian J. Biochem. Biophys. 1978, 21, 130–132. [Google Scholar]
- Aebi, H.; Vergmeyer, H.U. Catalase in Method Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; p. 673. [Google Scholar]
- Rotruck, J.; Pope, A.; Ganther, H.; Swanson, A. Selenium: Biochemical roles as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.; Pabst, M.; Jackoby, W. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- James, N.; Gay, R.; Hill, R. Influence of estrogen on glutathione levels and glutathione metabolizing enzymes in uteri and R3230 AC mammary tumors of rats. Biochem. Biophys. Acta 1980, 630, 485–496. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Bonting, S.L. Sodium-potassium activated adenosine triphosphatase and cation transport. Memb. Ion Transp. 1970, 1, 257–363. [Google Scholar]
- Scheff’e, H.A. A method for judging all contrasts in the analysis of variance. Biometrika 1953, 40, 87–104. [Google Scholar]
- Ahmed, M.B.; Khater, M.R. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. J. Ethnopharmacol. 2001, 75, 169–174. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Pandian, M.R. Evaluation of the antioxidant activity of Trianthema portulacastrum L. Indian J. Pharmacol. 2005, 37, 331–335. [Google Scholar] [CrossRef]
- Eaton, D.L.; Bammler, T.K. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol. Sci. 1999, 49, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, R.R. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J. Ethnopharmacol. 2003, 89, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Doss, A.; Anand, S.P. Free Radical Scavenging Activity of Solanum trilobatum Linn. on Alloxan-Induced Diabetic Rats. Biochem. Anal. Biochem. 2012, 1, 115. [Google Scholar]
| Treatment | Blood GSH (mg%) | Liver GSH (μmol/g of Liver) | Liver Na+K+ATPase (U/g prOtein) | TBARS (Nmol MDA/g of Wet Tissue/h) |
|---|---|---|---|---|
| Control | 2.82 ± 0.06 | 11.2 ± 0.56 | 13.6 ± 1.23 | 359.42 ± 18.45 |
| TAA | 0.98 ± 0.02 a | 7.89 ± 0.56 b | 8.56 ± 1.03 b | 498.5 ± 15.69 a |
| S. trilobatum (100 mg/kg bw) | 1.96 ± 0.04 c (53.3) | 8.95 ± 1.06 d (32) | 10.57 ± 1.13 c (40) | 409.6 ± 19.46 c (64) |
| S. trilobatum (200 mg/kg bw) | 2.36 ± 0.56 c (75) | 10.2 ± 1.23 c (70) | 12.65 ± 1.64 c (75.7) | 379.4 ± 19.49 c (85.6) |
| Treatment | GSR (µmol NADPH/min/g/of Wet Liver) | GPx (U/mg Protein) | GST (U/g of Wet Weight) | SOD (U/mg Protein) | CAT (H2O2 Decomposed/min/mg Protein) |
|---|---|---|---|---|---|
| Control | 189.5 ± 10.8 | 13.46 ± 1.78 | 156.4 ± 9.43 | 10.56 ± 1.89 | 90.25 ± 8.2 |
| TAA | 122.6 ± 12.8 a | 8.97 ± 1.02 b | 88.9 ± 5.6 b | 5.57 ± 0.96 a | 55.96 ± 8.7 a |
| S. trilobatum (100 mg/kg bw) | 155.8 ± 15.6 d (50) | 10.9 ± 1.46 d (43) | 116.9 ± 9.87 d (41.4) | 8.9 ± 1.72 d (66.7) | 72.8 ± 9.8 c (49.1) |
| S. trilobatum (200 mg/kg bw) | 169.2 ± 10.3 c (69.6) | 12.9 ± 1.08 c (87.5) | 134.6 ± 10.6 d (67.7) | 9.35 ± 0.27 c (75.7) | 86.4 ± 9.89 c (88.7) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Sukalingam, K.; Xu, B. Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats. Antioxidants 2017, 6, 68. https://doi.org/10.3390/antiox6030068
Ganesan K, Sukalingam K, Xu B. Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats. Antioxidants. 2017; 6(3):68. https://doi.org/10.3390/antiox6030068
Chicago/Turabian StyleGanesan, Kumar, Kumeshini Sukalingam, and Baojun Xu. 2017. "Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats" Antioxidants 6, no. 3: 68. https://doi.org/10.3390/antiox6030068
APA StyleGanesan, K., Sukalingam, K., & Xu, B. (2017). Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats. Antioxidants, 6(3), 68. https://doi.org/10.3390/antiox6030068