Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review
Abstract
:1. Introduction
Search Strategy
2. Phytochemical Compounds
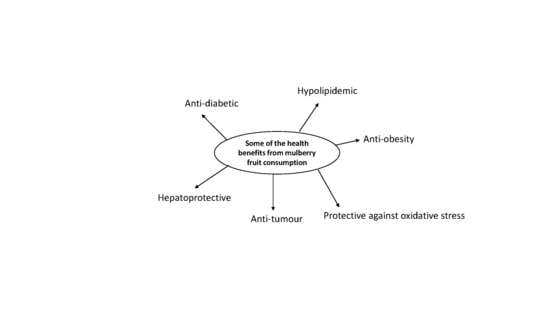
3. Pharmacological Properties
3.1. Hypolipidemic
3.2. Anti-Diabetic
3.3. Anti-Obesity
3.4. Anti-Tumour
3.5. Hepatoprotective
3.6. Protective against Cytotoxicity and Oxidative Stress
3.7. Protective against Brain Damage
3.8. Adverse Effects
4. Conclusions and Future Research
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Li, X.-J.; Zhang, H.-Y. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, Z.F.; Zhang, H.; Jin, Y.; Zhang, Y.; Hayford, F. Phytochemical properties and nutrigenomic implications of yacon as a potential source of prebiotic: Current evidence and future directions. Foods 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Ye, F.; Shen, Z.; Xie, M. Alpha-glucosidase inhibition from a Chinese medical herb (Ramulus mori) in normal and diabetic rats and mice. Phytomedicine 2002, 9, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, M.B.; Hossain, M.; Rashid, M.; Sadik, G.; Nasrin, S.; et al. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (moraceae). BMC Res. Notes 2013, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Lu, B.; Zhang, Y. The processing technology of mulberry series product. China Fruit Veg. Proc. 2005, 5, 38–40. [Google Scholar]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus Indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Sohn, B.-H.; Park, J.-H.; Lee, D.-Y.; Cho, J.-G.; Kim, Y.-S.; Jung, I.-S.; Kang, P.-D.; Baek, N.-I. Isolation and identification of lipids from the silkworm (Bombyx mori) droppings. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 336–341. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T.; Xiao, L.; Yang, L.; Chen, R. Two new chalcones from leaves of Morus alba L. Fitoterapia 2010, 81, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Park, S. Mulberry extract supplements ameliorate the inflammation-related hematological parameters in carrageenan-induced arthritic rats. J. Med. Food 2006, 9, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, L. Anti-inflammatory and analgesic properties of cis-mulberroside a from Ramulus mori. Fitoterapia 2010, 81, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Gerasopoulos, D.; Stavroulakis, G. Quality characteristics of four mulberry (Morus sp) cultivars in the area of Chania, Greece. J. Sci. Food Agric. 1997, 73, 261–264. [Google Scholar] [CrossRef]
- Suhl, H.J.; Noh, D.O.; Kang, C.S.; Kim, J.M.; Lee, S.W. Thermal kinetics of color degradation of mulberry fruit extract. Die Nahr. 2003, 47, 132–135. [Google Scholar]
- Liu, X.; Xiao, G.; Chen, W.; Xu, Y.; Wu, J. Quantification and purification of mulberry anthocyanins with macroporous resins. J. Biomed. Biotechnol. 2004, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Xu, Y.; Gowd, V.; Zhao, J.; Xie, J.; Liang, W.; Chen, W. Systematic study on phytochemicals and antioxidant activity of some new and common mulberry cultivars in China. J. Funct. Foods 2016, 25, 537–547. [Google Scholar] [CrossRef]
- Kusano, G.; Orihara, S.; Tsukamoto, D.; Shibano, M.; Coskun, M.; Guvenc, A.; Erdurak, C.S. Five new nortropane alkaloids and six new amino acids from the fruit of Morus alba Linne growing in Turkey. Chem. Pharm. Bull. 2002, 50, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic profiles, antioxidant activities, and neuroprotective properties of mulberry (Morus atropurpurea Roxb.) fruit extracts from different ripening stages. J. Food Sci. 2016, 81, C2439–C2446. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [PubMed]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-H.; Suh, H.-J. Antioxidant activities of five different mulberry cultivars in Korea. Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorgan. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef] [PubMed]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J. Food Sci. 2010, 28, 117–126. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Comp. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Memon, A.A.; Memon, N.; Luthria, D.L.; Bhanger, M.I.; Pitafi, A.A. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25–32. [Google Scholar]
- Peng, C.-H.; Liu, L.-K.; Chuang, C.-M.; Chyau, C.-C.; Huang, C.-N.; Wang, C.-J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.-B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, M.; Lee, B.L.; Ong, C.N.; Liu, X.; Huang, D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 2008, 56, 9410–9416. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiang, W.; Yu, Y.; Shi, Z.-Q.; Huang, X.-Z.; Xu, L. Comparative analysis of 1-deoxynojirimycin contribution degree to α-glucosidase inhibitory activity and physiological distribution in Morus alba L. Ind. Crops Prod. 2015, 70, 309–315. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.J.; Bucheli, P.; Zhang, P.F.; Wei, D.Z.; Lu, Y.H. Phytochemical profiles of different mulberry (Morus sp.) species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Bang, N.; Srichana, T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Calin-Sanchez, A.; Martinez-Nicolas, J.J.; Munera-Picazo, S.; Carbonell-Barrachina, A.A.; Legua, P.; Hernandez, F. Bioactive compounds and sensory quality of black and white mulberries grown in Spain. Plant Foods Hum. Nutr. 2013, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry fruit extract affords protection against ethyl carbamate-induced cytotoxicity and oxidative stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.Q.; Guo, Y.; Xu, D.H.; Huang, Y.S.; Yuan, K.; Lv, Z.Q. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem. Toxicol. 2013, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.T.; Perazzo, F.F.; Machado, L.; Bereau, D. Biologic activity and biotechnological development of natural products. Biomed. Res. Int. 2013, 971745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lila, M.A. Anthocyanins and human health: An in vitro investigative approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, B.L.; Li, Y. Advances in the pharmacological study of Morus alba L. Acta Pharm. Sin. 2014, 49, 824–831. [Google Scholar]
- Huang, H.P.; Ou, T.T.; Wang, C.J. Mulberry (sang shen zi) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Liu, L.-K.; Hsu, J.-D.; Huang, H.-P.; Yang, M.-Y.; Wang, C.-J. Mulberry extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Food Chem. 2005, 91, 601–607. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Yang, M.-Y.; Chen, S.-C.; Wang, C.-J. Mulberry leaf polyphenol extract improves obesity by inducing adipocyte apoptosis and inhibiting preadipocyte differentiation and hepatic lipogenesis. J. Funct. Foods 2016, 21, 249–262. [Google Scholar] [CrossRef]
- Kwon, H.J.; Chung, J.Y.; Kim, J.Y.; Kwon, O. Comparison of 1-deoxynojirimycin and aqueous mulberry leaf extract with emphasis on postprandial hypoglycemic effects: In vivo and in vitro studies. J. Agric. Food. Chem. 2011, 59, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-G.; Ji, D.-F.; Zhong, S.; Lv, Z.-Q.; Lin, T.-B.; Chen, S.; Hu, G.-Y. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J. Ethnopharmacol. 2011, 134, 961–970. [Google Scholar] [PubMed]
- Naowaratwattana, W.; De-Eknamkul, W.; De Mejia, E.G. Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase II alpha activity. J. Med. Food. 2010, 13, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Kukongviriyapan, U.; Nakmareong, S.; Itharat, A. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr. Res. 2009, 29, 602–608. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.M.; do Nascimento, M.F.; Ferreira, M.R.A.; de Moura, D.F.; dos Santos Souza, T.G.; da Silva, G.C.; da Silva Ramos, E.H.; Paiva, P.M.G.; de Medeiros, P.L.; da Silva, T.G.; et al. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (moraceae) in mice. J. Ethnopharmacol. 2016, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, R.; Zheng, N.; Xu, L.; Liang, T.; He, Q. Anti-diabetic effect of Ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int. Immunopharmacol. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Lee, Y.Y. Virgin coconut oil and its cardiovascular health benefits. Nat. Prod. Commun. 2016, 11, 1151–1152. [Google Scholar]
- Lu, H.; Pan, W.-Z.; Wan, Q.; Cheng, L.-L.; Shu, X.-H.; Pan, C.-Z.; Qian, J.-Y.; Ge, J.-B. Trends in the prevalence of heart diseases over a ten-year period from single-center observations based on a large echocardiographic database. J. Zhejiang Univ. Sci. B 2016, 17, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V. Single risk factor intervention may be inadequate to inhibit atherosclerosis progression when hypertension and hypercholesterolemia coexist. Hypertension 1991, 18, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Devaraj, S.N.; Devaraj, H. Increased binding of LDL and VLDL to apo B,E receptors of hepatic plasma membrane of rats treated with Fibernat. Eur. J. Nutr. 2003, 42, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Sirikanchanarod, A.; Bumrungpert, A.; Kaewruang, W.; Senawong, T.; Pavadhgul, P. The effect of mulberry fruits consumption on lipid profiles in hypercholesterolemic subjects: A randomized controlled trial. J. Pharm. Nutr. Sci. 2016, 60, 7–14. [Google Scholar]
- Kalofoutis, C.; Piperi, C.; Kalofoutis, A.; Harris, F.; Phoenix, D.; Singh, J. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp. Clin. Cardiol. 2007, 12, 17–28. [Google Scholar] [PubMed]
- Xu, L.; Yang, F.; Wang, J.; Huang, H.; Huang, Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015, 117, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Dai, G.; Zheng, X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 2016, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Jensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Castro Cabezas, M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Lee, S.O.; Kim, S.Y.; Yang, S.J.; Lim, Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp. Biol. Med. 2013, 238, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A.; Vahedi, M.; Baghestani, A.R. Burden of gastrointestinal cancer in Asia: An overview. Gastroenterol. Hepatol. Bed Bench 2015, 8, 19–27. [Google Scholar] [PubMed]
- Ma, Z.F.; Majid, N.A.; Yamaoka, Y.; Lee, Y.Y. Food allergy and helicobacter pylori infection: A systematic review. Front. Microbiol. 2016, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H. Gastric carcinogenesis and underlying molecular mechanisms: Helicobacter pylori and novel targeted therapy. BioMed Res. Int. 2015, 2015, 794378. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-P.; Chang, Y.-C.; Wu, C.-H.; Hung, C.-N.; Wang, C.-J. Anthocyanin-rich Mulberry extract inhibit the gastric cancer cell growth in vitro and xenograft mice by inducing signals of p38/p53 and c-jun. Food Chem. 2011, 129, 1703–1709. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Jia, S.; Yuan, K. Protective effect and mechanism of action of mulberry marc anthocyanins on carbon tetrachloride-induced liver fibrosis in rats. J. Funct. Foods 2016, 24, 595–601. [Google Scholar] [CrossRef]
- Chang, J.-J.; Hsu, M.-J.; Huang, H.-P.; Chung, D.-J.; Chang, Y.-C.; Wang, C.-J. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. Consort 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandis, N.; Fleming, P.S.; Hopewell, S.; Altman, D.G. The consort statement: Application within and adaptations for orthodontic trials. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, L. The mulberry (Morus alba L.) fruit—A review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical profile of brown rice and its nutrigenomic implication. Antioxidants 2018. accepted for publication. [Google Scholar]

| Pharmacological Properties | References |
|---|---|
| Hypolipidemic | Yang et al. [11]; Chen et al. [50]; Sirikanchanarod et al. [66] |
| Anti-diabetic | Wang et al. [15]; Jiao et al. [59]; Guo et al. [58]; Xu et al. [68]; Yan et al. [69] |
| Anti-obesity | Peng et al. [35]; Lim et al. [74] |
| Anti-tumour | Huang et al. [79] |
| Hepatoprotective | Li et al. [80]; Chang et al. [81] |
| Protective against cytotoxicity and oxidative stress | Jiang et al. [44]; Chang et al. [81] |
| Protective against brain damage | Kang et al.[17] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. https://doi.org/10.3390/antiox7050069
Zhang H, Ma ZF, Luo X, Li X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants. 2018; 7(5):69. https://doi.org/10.3390/antiox7050069
Chicago/Turabian StyleZhang, Hongxia, Zheng Feei Ma, Xiaoqin Luo, and Xinli Li. 2018. "Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review" Antioxidants 7, no. 5: 69. https://doi.org/10.3390/antiox7050069
APA StyleZhang, H., Ma, Z. F., Luo, X., & Li, X. (2018). Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants, 7(5), 69. https://doi.org/10.3390/antiox7050069