Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A2 Activity at Cytoplasmic pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Production of Recombinant Prdx6
2.3. Measurement of aiPLA2 Activity
2.4. Mass Spectroscopy (ESI- MS and LC-MS/MS)
2.5. Fluorescence Spectroscopy
2.6. Circular Dichroism (CD)
2.7. Statistical Analysis
3. Results
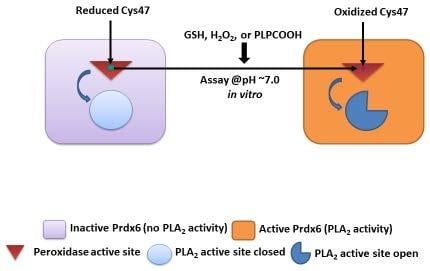
3.1. Effect of GSH on the PLA2 Activity of Prdx6
3.2. Molecular O2 is Required for Generation of the Sulfinic Prdx6
3.3. Prdx6 Modifications in the Presence of GSH Evaluated by ESI- MS and LC-MS/MS
3.4. GSH Induces Changes in Tryptophan Fluorescence and Circular Dichroism (CD) in hPrdx6
3.5. PLA2 Activity with Protein Modification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Dodia, C.; Chen, X.; Hennigan, B.B.; Jain, M.; Feinstein, S.I.; Fisher, A.B. Cloning and expression of rat lung acidic Ca2+-independent PLA2 and its organ distribution. Am. J. Physiol. Lung Cell. Mol. Physiol. 1998, 274, L750–L761. [Google Scholar] [CrossRef]
- Singh, A.K.; Shichi, H. A novel glutathione peroxidase in bovine eye. Sequence analysis, mRNA level, and translation. J. Biol. Chem. 1998, 273, 26171–26178. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Chen, J.W.; Dodia, C.; Feinstein, S.I.; Jain, M.K.; Fisher, A.B. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 2000, 275, 28421–28427. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Manevich, Y.; Chen, J.W.; Feinstein, S.I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J. Biol. Chem. 1999, 274, 21326–21334. [Google Scholar] [CrossRef]
- Manevich, Y.; Feinstein, S.I.; Fisher, A.B. Activation of the antioxidant enzyme 1-Cys peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. USA 2004, 101, 3780–3785. [Google Scholar] [CrossRef]
- Manevich, Y.; Fisher, A.B. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic. Biol. Med. 2005, 38, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Reddy, K.S.; Shuvaeva, T.; Feinstein, S.I.; Fisher, A.B. Structure and phospholipase function of peroxiredoxin 6: Identification of the catalytic triad and its role in phospholipid substrate binding. J. Lipid Res. 2007, 48, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel lysoPhosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J. Lipid Res. 2016, 57, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Feinstein, S.I.; Ho, Y.S. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J. Lipid Res. 2005, 46, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Akiba, S.; Dodia, C.; Chen, X.; Fisher, A.B. Characterization of acidic Ca2+-independent phospholipase A2 of bovine lung. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 393–404. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C. Role of phospholipase A2 enzymes in degradation of dipalmitoylphosphatidylcholine by granular pneumocytes. J. Lipid Res. 1996, 37, 1057–1064. [Google Scholar] [PubMed]
- Li, H.; Benipal, B.; Zhou, S.; Dodia, C.; Chatterjee, S.; Tao, J.Q.; Sorokina, E.M.; Raabe, T.; Feinstein, S.I.; Fisher, A.B. Critical role of peroxiredoxin 6 in the repair of peroxidized cell membranes following oxidative stress. Free Radic. Biol. Med. 2015, 87, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.B.; Vazquez-Medina, J.P.; Dodia, C.; Sorkina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase acvity in the repair of peroxidized cell membranes. Redox Biol. 2017, 14, 41–46. [Google Scholar] [CrossRef]
- Chatterjee, S.; Feinstein, S.I.; Dodia, C.; Sorokina, E.; Lien, Y.C.; Nguyen, S.; Debolt, K.; Speicher, D.; Fisher, A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011, 286, 11696–11706. [Google Scholar] [CrossRef]
- Vazquez-Medina, J.P.; Dodia, C.; Weng, L.; Mesaros, C.; Blair, I.; Feinstein, S.I.; Chatterjee, C.; Fisher, A. The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. FASEB J. 2016, 30, 2885–2898. [Google Scholar] [CrossRef]
- Wu, Y.; Feinstein, S.I.; Manevich, Y.; Chowdhury, I.; Pak, J.H.; Kazi, A.; Dodia, C.; Speicher, D.W.; Fisher, A.B. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A2 activity. Biochem. J. 2009, 419, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, H.; Zhou, S.; Dodia, C.; Feinstein, S.I.; Huang, S.; Speicher, D.; Fisher, A.B. Increased phospholipase A2 activity with phosphorylation of peroxiredoxin 6 requires a conformational change in the protein. Biochemistry 2012, 51, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Shuvaeva, T.; Dodia, C.; Kazi, A.; Feinstein, S.I.; Fisher, A.B. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities. Arch. Biochem. Biophys. 2009, 485, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jo, H.Y.; Kim, M.H.; Cha, Y.Y.; Choi, S.W.; Shim, J.H.; Kim, T.J.; Lee, K.Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008, 283, 33563–33568. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Manevich, Y.; Baldwin, J.L.; Dodia, C.; Yu, K.; Feinstein, S.I.; Fisher, A.B. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J. Biol. Chem. 2006, 281, 7515–7525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sorokina, E.; Harper, S.; Ralat, L.; Dodia, C.; Speicher, D.; Feinstein, S.I.; Fisher, A. Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity. Free Radic. Biol. Med. 2016, 94, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Sweitzer, T.; Pak, J.H.; Feinstein, S.I.; Muzykantov, V.; Fisher, A.B. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc. Natl. Acad. Sci. USA 2002, 99, 11599–11604. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Davies, M.J.; Kramer, A.C.; Miotto, G.; Zaccarin, M.; Zhang, H.; Ursini, F. Protein cysteine oxidation in redox signaling: Caveats on sulfenic acid detection and quantification. Arch. Biochem. Biophys. 2017, 617, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Academic/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Vivian, J.T.; Callis, P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Chander, A.; Jain, M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. Biochem. J. 1992, 288(Pt. 2), 407–411. [Google Scholar] [CrossRef]
- Jain, M.K.; Tao, W.J.; Rogers, J.; Arenson, C.; Eibl, H.; Yu, B.Z. Active-site-directed specific competitive inhibitors of phospholipase A2: Novel transition-state analogues. Biochemistry 1991, 30, 10256–10268. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, S.; Cummings, A.H.; Gates, K.S. Protection of a single-cysteine redox switch from oxidative destruction: On the functional role of sulfenyl amide formation in the redox-regulated enzyme PTP1B. Bioorg. Med. Chem. Lett. 2010, 20, 444–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albro, P.W.; Corbett, J.T.; Schroeder, J.L. Generation of hydrogen peroxide by incidental metal ion-catalyzed autooxidation of glutathione. J. Inorg. Biochem. 1986, 27, 191–203. [Google Scholar] [CrossRef]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Nagy, P.; Winterbourn, C. Advances in Molecular Toxicology. Redox Chem. Biol. Thiols 2010, 4, 183–222. [Google Scholar]
- Zhou, S.; Lien, Y.C.; Shuvaeva, T.; DeBolt, K.; Feinstein, S.I.; Fisher, A.B. Functional interaction of glutathione S-transferase pi and peroxiredoxin 6 in intact cells. Intern. J. Biochem. Cell. Biol. 2013, 45, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralat, L.A.; Manevich, Y.; Fisher, A.B.; Colman, R.F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry 2006, 45, 360–372. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, S.W.; Yang, C.H.; Rhee, S.G.; Ryu, S.E. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat. Struct. Biol. 1998, 5, 400–406. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, W.; Kim, E.E. Crystal structures of human peroxiredoxin 6 in different oxidation states. Biochem. Biophys. Res. Commun. 2016, 477, 717–722. [Google Scholar] [CrossRef]
- Rivera-Santiago, R.F.; Harper, S.L.; Zhou, S.; Sriswasdi, S.; Feinstein, S.I.; Fisher, A.B.; Speicher, D.W. Solution structure of the reduced form of human peroxiredoxin-6 elucidated using zero-length chemical cross-linking and homology modelling. Biochem. J. 2015, 468, 87–98. [Google Scholar] [CrossRef]





| Condition | PLA2 Activity at pH 7.4 nmol/min/mg | |
|---|---|---|
| −GSH | +GSH | |
| Control | 0.3 ± 0.06 (6) | 51 ± 1 (6) |
| +MJ33 | ___ | 19 ± 2* (3) |
| +BEL | ___ | 50 ± 1 (4) |
| +GSSG | 3.2 ± 2.1 (3) | __ |
| +DTT | 2.0 ± 1.4 (3) | __ |
| +TCEP | 0.5 ± 0.1 (3) | __ |
| Anaerobic | 0.2 ± 0.1 (4) | 0.2 ± 0.1* (4) |
| Liposomes with PLPC† | 0.3 ± 0.02 (6) | 48 ± 1 (6) |
| Oxidized liposomes with PLPC† | 100 ± 1* (3) | 100 ± 1* (3) |
| Phosphorylated Prdx6 | 1220 ± 6* (3) | 1150 ± 3* (3) |
| Oxidized Prdx6 | 100 ± 3* (3) | 99 ± 1* (3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Dodia, C.; Feinstein, S.I.; Harper, S.; Forman, H.J.; Speicher, D.W.; Fisher, A.B. Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A2 Activity at Cytoplasmic pH. Antioxidants 2019, 8, 4. https://doi.org/10.3390/antiox8010004
Zhou S, Dodia C, Feinstein SI, Harper S, Forman HJ, Speicher DW, Fisher AB. Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A2 Activity at Cytoplasmic pH. Antioxidants. 2019; 8(1):4. https://doi.org/10.3390/antiox8010004
Chicago/Turabian StyleZhou, Suiping, Chandra Dodia, Sheldon I. Feinstein, Sandra Harper, Henry J. Forman, David W. Speicher, and Aron B. Fisher. 2019. "Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A2 Activity at Cytoplasmic pH" Antioxidants 8, no. 1: 4. https://doi.org/10.3390/antiox8010004
APA StyleZhou, S., Dodia, C., Feinstein, S. I., Harper, S., Forman, H. J., Speicher, D. W., & Fisher, A. B. (2019). Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A2 Activity at Cytoplasmic pH. Antioxidants, 8(1), 4. https://doi.org/10.3390/antiox8010004