Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material and Sample Preparation
2.2. Isolation of Extracts Rich in Polyphenols from Mango and its By-Produts
2.2.1. Extracts for Anti-Platelet Aggregation Activity Assay
2.2.2. Extracts for Analytical Characterization
2.3. Human Platelet Isolation
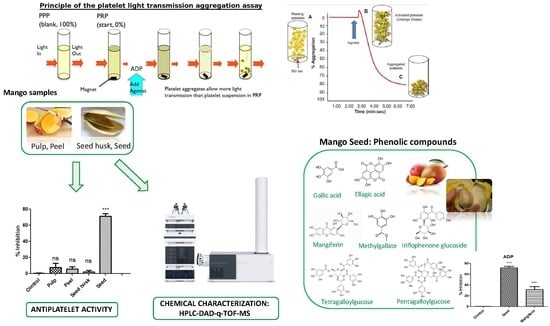
2.4. Anti-Platelet Aggregation Activity Assay
2.5. Phenolic Characterization of Extracts by HPLC-DAD-q-TOF-MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Anti-Platelet Aggregation Activity of Mango and its By-Products
3.2. Phenolic Characterization of Extracts by HPLC-DAD-q-TOF-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badimon, L.; Vilahur, G. Coronary Atherothrombotic Disease: Progress in Antiplatelet Therapy. Revista Esp. de Cardiol. (English Edition). 2008, 5, 501–513. [Google Scholar] [CrossRef]
- Davies, M.J. The pathophysiology of acute coronary syndromes. Heart 2000, 83, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Geisler, T.; Anders, N.; Paterok, M.; Langer, H.; Stellos, K.; Lindemann, S.; Herdeg, C.; May, A.E.; Gawaz, M. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 2007, 30, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xu, C.; He, Z.L.; Zhou, Q.M.; Wang, J.B.; Li, Z.Y.; Ming, X. A novel peptide inhibitor of platelet aggregation from stiff silkworm, Bombyx batryticatus. Peptides 2014, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Palomo, I. Antiplatelet effects of natural bioactive compounds by multiple targets: Food and drug interactions. J. Funct. Foods 2014, 6, 73–81. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction and possible utilization. Compr. Rev. Food Sci. F. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on Mango (Mangifera indica L.) kernel fat of some Kenyan varieties in Meru. J. Am. Oil Chem. Soc. 2012, 89, 1567–1575. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodríguez, J.A.; Wasim Siddiqui, M.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic by-products as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.A.I.; Rehman, H.; Aftab, S. Therapeutic potentials of bioactive compounds from mango fruit waste. Trends Food Sci. Tech. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Massibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. F. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Albuquerque Cavalacanti de Albuquerque, M.; Levit, R.; Beres, C.; Bedani, R.; De Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibers and phenolic compounds with potential antioxidant, anti-inflammatory and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.J.; Astudillo, L.A.; Gutierrez, M.I.; Contreras, S.O.; Bustamante, L.O.; Rubio, P.I.; Moore-Carrasco, R.; Alarcon, M.; Fuentes, A.; Gonzalez, J.A.; et al. Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood Coagul. Fibrinolysis 2012, 23, 109–117. [Google Scholar] [CrossRef]
- Sai Srinivas, S.H.; Maneesh Kumar, M.; Prasada Rao, U.J.S. Studies on isolation and antioxidant properties of bioactive phytochemicals from mango peel harvested at different developmental stages. Int. J. Agric. Environ. Bio-res. 2017, 2, 136–148. [Google Scholar]
- Siriamornpun, S.; Kaewseejan, N. Quality, bioactive compounds and antioxidant capacity of selected climateric fruits with relation to their maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins andhydrolyzable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Penta-galloylglucose (PGG): A valuable phenolic compounds with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Alañon, M.E.; Oliver-Simancas, R.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carrtero, A. Evolution of bioactive compounds of three mango cultivars (Mangifera indica L.) at different maturation stages analyzed by HPLC-DAD-q-TOF-MS. Food Res. Int. 2019, 125, 108526. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Quantification of gallic acid and ellagic (Mangifera indica L.) kernel and their effects onantioxidant activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies fo ellagic acid, a natural phenolic antioxidants. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Sroka, A.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and antiradical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Berardini, N.; Carle, R.; Schieber, A. Characterization of gallotannins and benzophenonederivates from mango (Mangifera indica L. cv. Tommy Atkins) peles, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Verardo, V.; Díaz de Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A. Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Quadri, F.; Telang, M.; Mandhare, A. Therapeutic and socmetic applications of mangiferin: An updated patent review (patents published after 2013). Expert Opin. Ther. Pat. 2019, 29, 463–479. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; You, X.; Li, C.; Zhang, E.; Li, Z.; Chen, G.; Peng, H. Phenolics and polysaccharides in major tropical fruits: Chemical compositions, analytical methods and bioactivities. Anal. Methods 2011, 3, 2212–2220. [Google Scholar] [CrossRef]
- Tesfaye, T. Valorisation of mango fruit by-products: Physicochemical characterization and future prospect. Chem. Process. Eng. Res. 2017, 50, 22–34. [Google Scholar]
- Nguyen, N.M.P.; Le, T.T.; Vissenaekens, H.; Gonzles, G.B.; Van Camp, J.; Smagghe, G.; Raes, K. In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. Int. J. Food Sci. Technol. 2019, 54, 1169–1178. [Google Scholar] [CrossRef]



| COMPOUNDS | PULP | PEEL | SEED HUSK | SEED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | MEAN | SD | MEAN | SD | |||||
| Monogalloyl compounds | ||||||||||||
| Gallic acid | 1.55 | ± | 0.02 a | 3.57 | ± | 0.47 b | 3.93 | ± | 0.24 b | 16.67 | ± | 0.03 c |
| Galloylglucose | 42.36 | ± | 0.23 a | 112.17 | ± | 1.10 c | 43.09 | ± | 1.83 a | 53.67 | ± | 4.73 b |
| Galloyl diglucoside | 0.09 | ± | 0.00 a | 5.69 | ± | 0.07 b | 0.25 | ± | 0.01a | 6.64 | ± | 0.11 c |
| Methylgallate | 5.41 | ± | 0.48 a | 54.14 | ± | 7.77 b | 14.43 | ± | 0.89 a | 558.86 | ± | 6.74 c |
| Galloylquinic acid | 2.76 | ± | 0.12 a | 59.77 | ± | 1.79 c | 6.45 | ± | 0.50 b | 84.46 | ± | 0.34 d |
| Digalloyl compounds | ||||||||||||
| Digallic acid | ND | 2.54 | ± | 0.34 a | 1.67 | ± | 0.00 a | 3.64 | ± | 0.57 b | ||
| Digalloylglucose | 4.59 | ± | 0.03 a | 31.17 | ± | 0.57 c | 9.18 | ± | 0.59 b | 4.23 | ± | 0.09 a |
| Methyl digallate | 3.33 | ± | 0.44 a | 27.80 | ± | 4.88 c | 10.47 | ± | 0.50 b | ND | ||
| Digalloylquinic acid | 0.22 | ± | 0.00 a | 76.48 | ± | 0.55 d | 1.18 | ± | 0.10 b | 2.22 | ± | 0.02 c |
| Gallotannins | ||||||||||||
| Trigalloylglucose | 0.02 | ± | 0.02 a | 9.07 | ± | 0.04 d | 0.85 | ± | 0.03 b | 3.26 | ± | 0.05 c |
| Tetragalloylglucose | 1.64 | ± | 0.02 a | 14.50 | ± | 0.89 b | 3.16 | ± | 0.13 a | 88.37 | ± | 3.22 c |
| Penta-galloylglucose | 4.13 | ± | 0.07 a | 26.61 | ± | 0.91 b | 3.93 | ± | 0.04 a | 177.31 | ± | 2.14 c |
| Hexagalloylglucose | 4.00 | ± | 0.08 a | 74.46 | ± | 0.03 b | 9.78 | ± | 0.25 a | 63.72 | ± | 7.92 b |
| Hepta-galloylglucose | 3.59 | ± | 0.00 a | 58.26 | ± | 1.75 c | 7.75 | ± | 0.15 b | ND | ||
| COMPOUNDS | PULP | PEEL | SEED HUSK | SEED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | MEAN | SD | MEAN | SD | |||||
| Vanillic acid glucoside | 3.90 | ± | 0.19 a | 16.68 | ± | 0.42 c | 7.94 | ± | 0.36 b | 15.91 | ± | 0.77 c |
| p-Hydroxybenzoic acid glucoside | 7.65 | ± | 0.52 c | 7.27 | ± | 0.20 c | 2.58 | ± | 0.07 a | 6.06 | ± | 0.18 b |
| Dihydroxybenzoic acid glucoside | 0.45 | ± | 0.00 a | 0.36 | ± | 0.01 a | 0.75 | ± | 0.07 b | ND | ||
| Hydroxybenzoyl galloyl glucoside | 0.26 | ± | 0.00 b | 0.26 | ± | 0.01 b | 0.07 | ± | 0.01 a | ND | ||
| Coumaric acid glucoside | 0.07 | ± | 0.00 a | 0.52 | ± | 0.01 c | 0.29 | ± | 0.02 b | ND | ||
| Coumaroyl galloyl glucoside | 0.29 | ± | 0.02 a,b | 19.69 | ± | 0.27 c | 0.61 | ± | 0.00 b | ND | ||
| Ferulic acid hexoside | 2.50 | ± | 0.09 b | 1.81 | ± | 0.01 a | 1.91 | ± | 0.01 a | 2.91 | ± | 0.22 c |
| Sinapic acid hexoside | 0.32 | ± | 0.00 a | 0.97 | ± | 0.00 b | ND | ND | ||||
| Sinapic acid hexoside-pentoside | 7.73 | ± | 0.08 b | 14.16 | ± | 0.07 c | 6.96 | ± | 0.05 a | ND | ||
| Dihydro sinapic acid hexoside-pentoside | 3.57 | ± | 0.07 a | 4.90 | ± | 0.08 b | 3.53 | ± | 0.09 a | ND | ||
| COMPOUNDS | PULP | PEEL | SEED HUSK | SEED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | MEAN | SD | MEAN | SD | |||||
| Ellagic acid | 0.02 | ± | 0.00 a | 1.35 | ± | 0.03 b | 0.56 | ± | 0.04 a | 7.46 | ± | 0.54 c |
| Catechin | 0.49 | ± | 0.01 a | 12.18 | ± | 0.78 c | 1.17 | ± | 0.26 a | 9.97 | ± | 0.71 b |
| Quercetin glucoside | ND | 21.28 | ± | 0.63 c | 0.21 | ± | 0.05 a | 1.39 | ± | 0.14 b | ||
| Quercetin galactoside | ND | 10.91 | ± | 0.45 b | 0.02 | ± | 0.00 a | ND | ||||
| Quercetin xyloside | ND | 2.83 | ± | 0.25 a | ND | ND | ||||||
| Quercetin arabinopyranoside | ND | 2.94 | ± | 0.20 a | ND | ND | ||||||
| Rhamnetin hexoside | ND | 2.37 | ± | 0.05 a | ND | ND | ||||||
| 7-O-galloyltricetilflavan | ND | 2.91 | ± | 0.11 a | ND | 13.99 | ± | 0.29 b | ||||
| Mangiferin | ND | ND | 3.75 | ± | 0.29 a | 148.12 | ± | 0.74 b | ||||
| Maclurin C-glucoside | ND | ND | 0.01 | ± | 0.01 a | 11.40 | ± | 0.48 b | ||||
| Maclurin galloyl glucoside | ND | 4.60 | ± | 0.04 b | 0.84 | ± | 0.12 a | 9.63 | ± | 0.04 c | ||
| Maclurin digalloyl glucoside | ND | 1.63 | ± | 0.04 a | ND | 6.21 | ± | 0.28 b | ||||
| Iriflophenone glucoside | ND | ND | ND | 10.89 | ± | 0.28 a | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products. Antioxidants 2019, 8, 517. https://doi.org/10.3390/antiox8110517
Alañón ME, Palomo I, Rodríguez L, Fuentes E, Arráez-Román D, Segura-Carretero A. Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products. Antioxidants. 2019; 8(11):517. https://doi.org/10.3390/antiox8110517
Chicago/Turabian StyleAlañón, María Elena, Iván Palomo, Lyanne Rodríguez, Eduardo Fuentes, David Arráez-Román, and Antonio Segura-Carretero. 2019. "Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products" Antioxidants 8, no. 11: 517. https://doi.org/10.3390/antiox8110517
APA StyleAlañón, M. E., Palomo, I., Rodríguez, L., Fuentes, E., Arráez-Román, D., & Segura-Carretero, A. (2019). Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products. Antioxidants, 8(11), 517. https://doi.org/10.3390/antiox8110517