Paraoxonase 1, HDL Subclasses and Post Surgery Acute Inflammation: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Methods
2.2.1. PON1 Activity
Arylesterase
Lactonase
2.2.2. HDL Subfractions
2.3. Statistical Analysis
3. Results
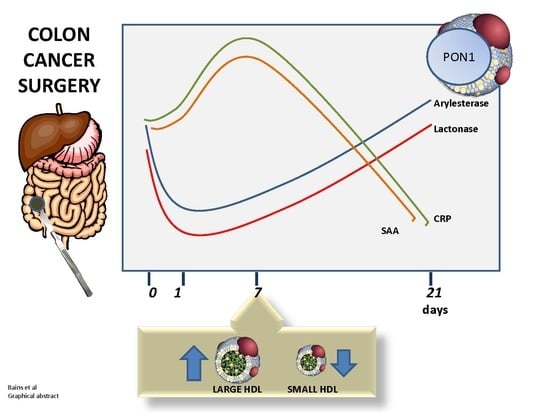
3.1. Baseline PON1 Activity is Lower in Colon Cancer Patients
3.2. Significant Further Reduction in PON1 Activity Was Observed Post-Surgery
3.3. PON1 Changes Are Negatively Associated with Changes in Other Markers of Inflammation
3.4. Small and Intermediate HDL Decrease in the Hyperacute Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 224, pp. 3–51. [Google Scholar]
- Albers, J.J.; Slee, A.; Fleg, J.L.; O’Brien, K.D.; Marcovina, S.M. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis 2016, 251, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Burt, A.A.; Rosenthal, E.A.; Ranchalis, J.E.; Eintracht, J.F.; Hatsukami, T.S.; Furlong, C.E.; Marcovina, S.; Albers, J.J.; Jarvik, G.P. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J. Am. Heart Assoc. 2014, 3, e000902. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Pérez-Méndez, Ó.; Pacheco, H.G.; Martínez-Sánchez, C.; Franco, M. HDL-cholesterol in coronary artery disease risk: Function or structure? Clin. Chim. Acta 2014, 429, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. HDL particle number and size as predictors of cardiovascular disease. Front. Pharmacol. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.; Aviram, M.; Khatib, S.; Volkova, N.; Vaya, J. Human carotid atherosclerotic plaque protein(s) change HDL protein(s) composition and impair HDL anti-oxidant activity. BioFactors 2016, 42, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Birner-Gruenberger, R.; Schittmayer, M.; Holzer, M.; Marsche, G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog. Lipid Res. 2014, 56, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Mackness, M. The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med. 2012, 54, 83–90. [Google Scholar]
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Gaidukov, L.; Tawfik, D.S. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry 2005, 44, 11843–11854. [Google Scholar] [CrossRef]
- Aviram, M.; Vaya, J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr. Opin. Lipidol. 2013, 24, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, S.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013, 228, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Billecke, S.; Draganov, D.; Counsell, R.; Stetson, P.; Watson, C.; Hsu, C.; La Du, B.N. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 2000, 28, 1335–1342. [Google Scholar] [PubMed]
- Kotani, K.; Yamada, T.; Gugliucci, A. Paired measurements of paraoxonase 1 and serum amyloid A as useful disease markers. BioMed Res. Int. 2013, 2013, 481437. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc. Res. 2005, 68, 353–354. [Google Scholar] [CrossRef]
- Gugliucci, A. Activation of paraoxonase 1 is associated with HDL remodeling ex vivo. Clin. Chim. Acta 2014, 429, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Menini, T. Paraoxonase 1 and HDL maturation. Clin. Chim. Acta 2015, 439, 5–13. [Google Scholar] [CrossRef]
- Han, C.Y.; Chiba, T.; Campbell, J.S.; Fausto, N.; Chaisson, M.; Orasanu, G.; Plutzky, J.; Chait, A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1806–1813. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kotani, K.; Kimura, S. Paraoxonase 1 in Chronic Kidney Failure. J. Lipids 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Arenas, M.; Rodríguez, E.; Sahebkar, A.; Sabater, S.; Rizo, D.; Pallisé, O.; Hernández, M.; Riu, F.; Camps, J.; Joven, J. Paraoxonase-1 activity in patients with cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 127, 6–14. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kinugasa, E.; Ogata, H.; Caccavello, R.; Kimura, S. Activation of paraoxonase 1 after hemodialysis is associated with HDL remodeling and its increase in the HDL2 fraction and VLDL. Clin. Chim. Acta 2014, 430, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kappelle, P.J.W.H.; Bijzet, J.; Hazenberg, B.P.; Dullaart, R.P.F. Lower Serum Paraoxonase-1 Activity Is Related to Higher Serum Amyloid A Levels in Metabolic Syndrome. Arch. Med. Res. 2011, 42, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Luo, K.; Liu, H.; Huang, T.; Fang, X.; Tu, W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren. Fail. 2015, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Aviram, M.; Khatib, S.; Artoul, F.; Rabin, A.; Mannheim, D.; Karmeli, R.; Salamon, T.; Vaya, J. Human carotid plaque phosphatidylcholine specifically interacts with paraoxonase 1, increases its activity, and enhances its uptake by macrophage at the expense of its binding to HDL. Free Radic. Biol. Med. 2014, 76, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Hama, S.Y.; de Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; Escribano, A.; Díez-Sans, A.; Albiciuc, I.; Hernández-Aguilera, A.; Fort-Gallifa, I.; López-Azcona, A.F.; Camps, J.; Joven, J.; Castro, A. Influence of Surgical Procedures on Serum Paraoxonase-1-Related Variables and Markers of Inflammation in Hospitalized Patients. J. Investig. Surg. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shekhanawar, M.; Shekhanawar, S.M.; Krisnaswamy, D.; Indumati, V.; Satishkumar, D.; Vijay, V.; Rajeshwari, T.; Amareshwar, M. The role of ‘paraoxonase-1 activity’ as an antioxidant in coronary artery diseases. J. Clin. Diagn. Res. 2013, 7, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Jayakumari, N.; Thejaseebai, G. High Prevalence of Low Serum Paraoxonase-1 in Subjects with Coronary Artery Disease. J. Clin. Biochem. Nutr. 2009, 45, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abelló, D.; Sancho, E.; Camps, J.; Joven, J. Exploring the role of paraoxonases in the pathogenesis of coronary artery disease: A systematic review. Int. J. Mol. Sci. 2014, 15, 20997–21010. [Google Scholar] [CrossRef] [PubMed]
- Cabana, V.G.; Reardon, C.A.; Feng, N.; Neath, S.; Lukens, J.; Getz, G.S. Serum paraoxonase: Effect of the apolipoprotein composition of HDL and the acute phase response. J. Lipid Res. 2003, 44, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 123, 3815–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocka, A.; Cybulski, M.; Wysokiński, A.P.; Berbeć, H.; Stążka, J.; Zapolski, T. Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery. J. Clin. Med. 2019, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, Dense HDL Particles Exert Potent Protection of Atherogenic LDL Against Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchová, J.; Andrezálová, L.; Oravec, S.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z. High density lipoprotein subfractions and paraoxonase 1 in children. Acta Biochim. Pol. 2016, 63, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Otvos, J.D.; James, R.W. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in Type 2 diabetic and non-diabetic subjects. Clin. Biochem. 2014, 47, 1022–1027. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, Y.; Caccavello, R.; Kotani, K.; Gugliucci, A. Paraoxonase 1, HDL Subclasses and Post Surgery Acute Inflammation: A Pilot Study. Antioxidants 2019, 8, 192. https://doi.org/10.3390/antiox8060192
Bains Y, Caccavello R, Kotani K, Gugliucci A. Paraoxonase 1, HDL Subclasses and Post Surgery Acute Inflammation: A Pilot Study. Antioxidants. 2019; 8(6):192. https://doi.org/10.3390/antiox8060192
Chicago/Turabian StyleBains, Yasmin, Russell Caccavello, Kazuhiko Kotani, and Alejandro Gugliucci. 2019. "Paraoxonase 1, HDL Subclasses and Post Surgery Acute Inflammation: A Pilot Study" Antioxidants 8, no. 6: 192. https://doi.org/10.3390/antiox8060192
APA StyleBains, Y., Caccavello, R., Kotani, K., & Gugliucci, A. (2019). Paraoxonase 1, HDL Subclasses and Post Surgery Acute Inflammation: A Pilot Study. Antioxidants, 8(6), 192. https://doi.org/10.3390/antiox8060192