Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of in Vitro Antioxidant Potential by Inhibition of ABTS●+ Radical
2.2. Evaluation of In Vitro Antioxidant Potential Measured against the Production of Hydroxyl Radical
2.3. Evaluation of In Vitro Antioxidant Potential by Inhibition of DPPH Radical
2.4. Evaluation of the Qualitative Inhibition of the Enzyme Acetylcholinesterase
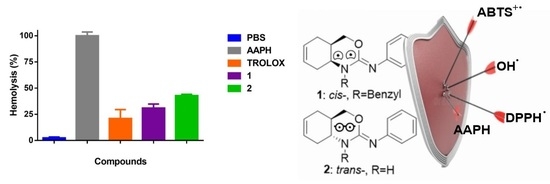
2.5. Test of Hemolysis Inhibition in Erythrocytes of Rattus norvegicus Induced by AAPH
2.6. Determination of Redox Potential
2.7. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Potential against the Production of Hydroxyl Radical
3.2. In Vitro Antioxidant Potential by Inhibition of ABTS●+ Radical
3.3. Hemolysis Inhibition in Erythrocytes of Rattus norvegicus Induced by AAPH and Qualitative Inhibition of the Enzyme Acetylcholinesterase
3.4. Cyclic Voltammetry
3.5. In Vitro Antioxidant Potential by Inhibition of DPPH● Radical
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, K.C.; Oliveira, G.L.S.; De Sousa, É.B.V.; Costa, I.H.F.; Machado, K.C.; De Sousa, D.P.; Satyal, P.; De Freitas, R.M. Spectroscopic studies on the in vitro antioxidant capacity of isopentyl ferulate. Chem.-Biol. Interact. 2015, 225, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhorukov, A.Y.; Nirvanappa, A.C.; Swamy, J.; Ioffe, S.L.; Swamy, S.N.; Basappa; Rangappa, K.S. Synthesis and characterization of novel 1,2-oxazine-based small molecules that targets acetylcholinesterase. Bioorg. Med. Chem. Lett. 2014, 24, 3618–3621. [Google Scholar] [CrossRef] [PubMed]
- Mendes de Freitas, R.; Da Silva Oliveira, G.L.; De Asevedo Mendes de Oliveira, F.R.; Oliveira Barros de Alencar, M.V.; Gomes Junior, A.L.; Araujo de Souza, A.; De Carvalho Melo Cavalcante, A.A. Evaluation of antioxidant capacity of the aqueous extract of Cynara scolymus L. (Asteraceae) in vitro and in Saccharomyces cerevisiae. Afr. J. Pharm. Pharmacol. 2014, 7, 136–147. [Google Scholar] [CrossRef]
- Alves, C.Q.; David, J.M.; David, J.P.; Bahia, M.V.; Aguiar, R.M. Methods for determination of in vitro antioxidant activity for extracts and organic compounds. Quím. Nova. 2010, 33, 2202–2210. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sadeghi, S.; Modarresi, M.; Abiri, R.; Mikaeli, A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem. Toxicol. 2010, 48, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Rohenkohl, C.C.; Carniel, A.P.; Colpo, E. Consumo de antioxidantes durante tratamento quimioterápico. ABCD Arq. Bras. Cir. Dig. (São Paulo) 2011, 24, 107–112. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Nogueira Neto, J.D.; De Almeida, A.A.C.; Da Silva Oliveira, J.; Dos Santos, P.S.; De Sousa, D.P.; De Freitas, R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Ansari, N.; Khodagholi, F.; Amini, M. 2-Ethoxy-4,5-diphenyl-1,3-oxazine-6-one activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Eur. J. Pharmacol. 2011, 658, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, R.; Kulshrestha, V.; Sankar, S.; Jubie, S. Docking studies, synthesis, characterization of some novel oxazine substituted 9-anilinoacridine derivatives and evaluation for their antioxidant and anticancer activities as topoisomerase II inhibitors. Eur. J. Med. Chem. 2012, 56, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Parlman, R.M.; Burns, L.D. Substituted Dihydro Oxazines as Hydrocarbon Antioxidants. U.S. Patent 4,313,738, 2 February 1982. [Google Scholar]
- Fülöp, F.; Bernáth, G.; Csirinyi, G. Synthesis of stereoisomers 2-phenylimino-3, 1-perhydro-benzoxazines and 3, 1-perhydrobenzothiazines. Org. Prep. Proced. Int. 1988, 20, 73–82. [Google Scholar] [CrossRef]
- Fülöp, F.; Bernáth, G.; Pihlaja, K. Synthesis, Stereochemistry and Transformations of Cyclopentane-, Cyclohexane-, Cycloheptane-, and Cyclooctane-Fused 1,3-Oxazines, 1,3-Thiazines, and Pyrimidines. Adv. Heterocycl. Chem. 1997, 69, 349–477. [Google Scholar] [CrossRef]
- Peláez, W.J.; Szakonyi, Z.; Fülöp, F.; Yranzo, G.I. Flash vacuum pyrolysis (fvp) of some hexahydroquinazolin-4(1H)-ones. Tetrahedron 2008, 64, 1049–1057. [Google Scholar] [CrossRef]
- Peláez, W.J.; Iriarte, A.G.; Szakonyi, Z.; Fülöp, F.; Argüello, G.A. Theoretical and experimental study on the reaction route for the FVP of 2-thioxohexahydroquinazolinones. J. Anal. Appl. Pyrolysis. 2012, 96, 181–187. [Google Scholar] [CrossRef]
- Iriarte, A.G.; Peláez, W.J.; Fülöp, F.; Argüello, G.A. Vibrational spectra of solid cis- and trans-2-thioxohexahydroquinazolin-4(1H)-one and theoretical calculations towards the interpretation of its thermal reactivity. RSC Adv. 2015, 5, 43345–43352. [Google Scholar] [CrossRef]
- De Brito, M.R.M.; Peláez, W.J.; Faillace, M.S.; Militão, G.C.G.; Almeida, J.R.G.S.; Argüello, G.A.; Szakonyi, Z.; Fülöp, F.; Salvadori, M.C.; Teixeira, F.S.; et al. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicol. Vitro. 2017, 44, 273–279. [Google Scholar] [CrossRef]
- Sawant, R.L.; Bhangale, L.; Wadekar, J.; Gaikwad, P. Substituent selection for design and synthesis of antimicrobial 1,3 oxazines: A topliss modified approach. Farmacia 2012, 60, 32–39. [Google Scholar]
- Sawant, R.L.; Mhaske, M.S.; Wadekar, J.B. Anticoagulant potential of Schiff bases of 1,3-oxazines. Int. J. PharmTech Res. 2012, 4, 320–323. [Google Scholar]
- Didwagh, S.S.; Piste, P.B. Novel one-pot Synthesis and Antimicrobial Activity of derivatives. Int. J. ChemtTech Res. 2013, 5, 2199–2203. [Google Scholar]
- Mathew, B.P.; Kumar, A.; Sharma, S.; Shukla, P.K.; Nath, M. An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo- and naphtho-1,3-oxazine derivatives. Eur. J. Med. Chem. 2010, 45, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, T.; Arikkatt, S.; Vincet, G.; Chandran, M.; Bhat, A.; Krishnakumar, K. Biological activities of oxazine and its derivatives. Int. J. Pharma Sci. Res. (IJPSR) 2013, 4, 134–143. [Google Scholar]
- Fülöp, F.; Szakonyi, Z.; Pallai, V.P. 1,3-Heterocycles Condensed with Monoterpene Skeleton, Their Use and Pharmaceutical Compositions Comprising Such Compounds. WO2010070365A1 (81), 24 June 2010. [Google Scholar]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst. 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions (This study is dedicated to the memory of Botany Professor Luiz F.G. Labouriau (1921–1996).). Biochim. Biophys. Acta. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rhee, I.K.; Van De Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pohanka, M. and Dobes, P. Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Torres, J.L.; Vinardell, M.P. Biobased epicatechin conjugates protect erythrocytes and nontumoral cell lines from H2O2-induced oxidative stress. J. Agric. Food Chem. 2009, 57, 4459–4465. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Mendonça, I. Coordinator of the Commission on Ethics in the Use of Animals (CEUA). 2016. Available online: https://ufpi.br/etica-em-experimentacao-animal (accessed on 12 June 2019).
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellad, J.M. Comparison of the simple cyclic voltammetry (CV) and DPPH assays for the determination of antioxidant capacity of active principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Hernández-Hierro, J.M.; Heredia, F.J.; Hernanz, D. Cyclic voltammetry to evaluate the antioxidant potential in winemaking by-products. Talanta 2017, 165, 211–215. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Miceli, M.; Roma, E.; Rosa, P.; Feroci, M.; Loreto, M.A.; Tofani, D.; Gasperi, T. Synthesis of Benzofuran-2-one derivatives and evaluation of their antioxidant capacity by comparing DPPH assay and cyclic voltammetry. Molecules 2018, 23, 710. [Google Scholar] [CrossRef]
- Sharma, V.; Jaiswal, P.K.; Saran, M.; Yadav, D.K.; Saloni; Mathur, M.; Swami, A.K.; Misra, S.; Kim, M.; Chaudhary, S. Discovery of C-3 Tethered 2-oxo-benzo[1,4]oxazines as Potent Antioxidants: Bio-Inspired Based Design, Synthesis, Biological Evaluation, Cytotoxic, and in Silico Molecular Docking Studies. Front. Chem. 2018, 6, 1–16. [Google Scholar] [CrossRef]
- Silva, C.G.; Herdeiro, R.S.; Mathias, C.J.; Panek, A.D.; Silveira, C.S.; Rodrigues, V.P.; Rennó, M.N.; Falcão, D.Q.; Cerqueira, D.M.; Minto, A.B.M.; et al. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Deng, J.; Cheng, W.; Yang, G. A novel antioxidant activity index (AAU) for natural products using the DPPH assay. Food Chem. 2011, 125, 1430–1435. [Google Scholar] [CrossRef]
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011, 51, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, K.; Arasteh, O.; Riahi, M.M.; Mehrid, S.; Riahi-Zanjani, B.; Karimi, G. Protective effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ. Toxicol. Pharmacol. 2014, 38, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Girish, T.K.; Vasudevaraju, P.; Prasada Rao, U.J.S. Protection of DNA and erythrocytes from free radical induced oxidative damage by black gram (Vigna mungo L.) husk extract. Food Chem. Toxicol. 2012, 50, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.; Ruberto, G. Kinetic solvent effects on phenolic antioxidants determined by spectrophotometric measurements. J. Agric. Food Chem. 2001, 49, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Foti, M. Use and Abuse of the DPPH● Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]







| Compound | Antioxidant Capacity (%) | ||||
|---|---|---|---|---|---|
| 9.0 μg mL−1 | 13.5 μg mL−1 | 18.0 μg mL−1 | 22.5 μg mL−1 | 27.0 μg mL−1 | |
| 1 | 71 ± 3 | 76.4 ± 0.2 | 76.9 ± 0.1 | 77.8 ± 0.1 | 78.5 ± 0.3 |
| 2 | 69.1 ± 0.3 | 79.2 ± 0.1 | 79.2 ± 0.3 | 79.4 ± 0.2 | 79.6 ± 0.1 |
| Trolox | 24 ± 1 | 40.3 ± 0.3 | 60 ± 5 | 62 ± 6 | 82 ± 2 |
| Compound | Antioxidant Capacity (%) | ||||
|---|---|---|---|---|---|
| 9.0 μg mL−1 | 13.5 μg mL−1 | 18.0 μg mL−1 | 22.5 μg mL−1 | 27.0 μg mL−1 | |
| 1 | 32.0 ± 0.4 | 36.4 ± 1 | 39.8 ± 0.4 | 42 ± 1 | 40.8 ± 0.5 |
| 2 | 38 ± 3 | 46 ± 4 | 46 ± 2 | 46 ± 1 | 46.6 ± 0.2 |
| Trolox | 38 ± 1 | 36.5 ± 0.5 | 37.7 ± 0.3 | 41.0 ± 0.6 | 41.3 ± 0.6 |
| Entry | Compound | Spectroscopic Analysis | Voltammetric Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH● | ABTS●+ | CV | ||||||||
| rEC50 | ARP | N° of reduced DPPH● | rEC50 | ARP | N° of reduced ABTS●+ | Eap (V) | Ecp (V) | ∆Ep(V) = |Ecp − Eap| | ||
| 1 | Trolox | 0.204 | 4.9 | 2.4 | 0.30 | 3.3 | 1.6 | 0.123 | 0.047 | 0.076 |
| 2 | 1 | 0 | 0 | 0 | 0.45 | 2.2 | 1.1 | 0.420 | −0.559 | 0.979 |
| 3 | 2 | 0 | 0 | 0 | 0.34 | 2.9 | 1.5 | 0.380 | −0.559 | 0.939 |
| 4 | AAc a | 0.227 | 4.4 | 2.2 | -- b | -- b | -- b | 0.322 | -- c | -- c |
| 5 | HQSAc a | 0.279 | 3.6 | 1.8 | -- b | -- b | -- b | 0.367 | −0.094 | 0.273 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firpo, G.; Ramírez, M.L.; Faillace, M.S.; de Brito, M.d.R.M.; e Silva, A.P.S.C.L.; Costa, J.P.; Rodríguez, M.C.; Argüello, G.A.; Szakonyi, Z.; Fülöp, F.; et al. Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines. Antioxidants 2019, 8, 197. https://doi.org/10.3390/antiox8060197
Firpo G, Ramírez ML, Faillace MS, de Brito MdRM, e Silva APSCL, Costa JP, Rodríguez MC, Argüello GA, Szakonyi Z, Fülöp F, et al. Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines. Antioxidants. 2019; 8(6):197. https://doi.org/10.3390/antiox8060197
Chicago/Turabian StyleFirpo, Guadalupe, María L. Ramírez, Martín S. Faillace, Maria dos R. Mendes de Brito, Ana P. S. Correia Lima e Silva, Jessica Pereira Costa, Marcela C. Rodríguez, Gustavo A. Argüello, Zsolt Szakonyi, Ferenc Fülöp, and et al. 2019. "Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines" Antioxidants 8, no. 6: 197. https://doi.org/10.3390/antiox8060197
APA StyleFirpo, G., Ramírez, M. L., Faillace, M. S., de Brito, M. d. R. M., e Silva, A. P. S. C. L., Costa, J. P., Rodríguez, M. C., Argüello, G. A., Szakonyi, Z., Fülöp, F., & Peláez, W. J. (2019). Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines. Antioxidants, 8(6), 197. https://doi.org/10.3390/antiox8060197