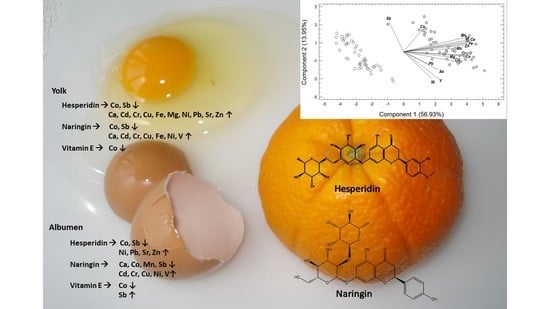
Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Sample Selection, and Preparation
2.2. Determination of Egg Trace and Macro Elements
2.3. Statistical Analysis
3. Results
3.1. Concentration of Egg Elements
3.2. Principal Components Analysis
3.3. Discriminant Analysis
4. Discussion
4.1. Flavonoids in Poultry Nutrition
4.2. Alteration of Egg Metallome
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pfaender, S.; Grabrucker, A.M. Characterization of biometal profiles in neurological disorders. Metallomics 2014, 6, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Szpunar, J. Metallomics: A new frontier in analytical chemistry. Anal. Bioanal. Chem. 2004, 378, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Danezis, G.; Pappas, A.; Zoidis, E.; Papadomichelakis, G.; Hadjigeorgiou, I.; Zhang, P.; Brusic, V.; Georgiou, C. Game meat authentication through rare earth elements fingerprinting. Anal. Chim. Acta 2017, 991, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Georgiou, C.A.; Brusic, V. Elemental metabolomics. Brief. Bioinform. 2017, 19, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Goetzke, B.; Nitzko, S.; Spiller, A. Consumption of organic and functional food. A matter of well-being and health? Appetite 2014, 77, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Karadas, F.; Grammenidis, E.; Surai, P.; Acamovic, T.; Sparks, N. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br. Poult. Sci. 2006, 47, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Elkin, R.G.; Ying, Y.; Harvatine, K.J. Feeding laying hens stearidonic acid-enriched soybean oil, as compared to flaxseed oil, more efficiently enriches eggs with very long-chain n-3 polyunsaturated fatty acids. J. Agric. Food Chem. 2015, 63, 2789–2797. [Google Scholar] [CrossRef]
- Laudadio, V.; Ceci, E.; Lastella, N.M.; Tufarelli, V. Dietary high-polyphenols extra-virgin olive oil is effective in reducing cholesterol content in eggs. Lipids Health Dis. 2015, 14, 5. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen egg as an antioxidant food commodity: A review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef]
- Sumaiya, S.; Nayak, S.; Baghel, R.; Nayak, A.; Malapure, C.; Kumar, R. Effect of dietary iodine on production of iodine enriched eggs. Vet. World 2016, 9, 554. [Google Scholar] [CrossRef]
- Tufarelli, V.; Ceci, E.; Laudadio, V. 2-Hydroxy-4-methylselenobutanoic acid as new organic selenium dietary supplement to produce selenium-enriched eggs. Biol. Trace Elem. Res. 2016, 171, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Čobanová, K.; Petrovič, V.; Mellen, M.; Arpášova, H.; Grešáková, Ľ.; Faix, Š. Effects of dietary form of selenium on its distribution in eggs. Biol. Trace Elem. Res. 2011, 144, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [PubMed]
- Zhang, J.; Brodbelt, J.S.; Wang, J. Threshold dissociation and molecular modeling of transition metal complexes of flavonoids. J. Am. Soc. Mass Spectrom. 2005, 16, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to a combination of diosmin, troxerutin and hesperidin and maintenance of normal venous-capillary permeability pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3511. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K. Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin In Vitro. Chem. Biol. Interact. 2011, 190, 121–128. [Google Scholar] [CrossRef]
- Lien, T.F.; Yeh, H.S.; Su, W.T. Effect of adding extracted hesperetin, naringenin and pectin on egg cholesterol, serum traits and antioxidant activity in laying hens. Arch. Anim. Nutr. 2008, 62, 33–43. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; Jordán, M.; López-Campos, Ó.; Giráldez, F.J.; Morán, L.; Andrés, S. The liver antioxidant status of fattening lambs is improved by naringin dietary supplementation at 015% rates but not meat quality. Animal 2012, 6, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Goliomytis, M.; Kartsonas, N.; Charismiadou, M.A.; Symeon, G.K.; Simitzis, P.E.; Deligeorgis, S.G. The influence of naringin or hesperidin dietary supplementation on broiler meat quality and oxidative stability. PLoS ONE 2015, 10, e0141652. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficay of naringin when used as a sensory additive for all animal species. EFSA J. 2011, 9, 2416. [Google Scholar] [CrossRef]
- Khan, M.H.A.; Parvez, S. Hesperidin ameliorates heavy metal induced toxicity mediated by oxidative stress in brain of Wistar rats. J. Trace Elem. Med. Biol. 2015, 31, 53–60. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Flavouring Group Evaluation 32 (FGE32) Flavonoids (Flavanones and dihydrochalcones) from chemical groups 25 and 30. EFSA J. 2010, 8, 1065. [Google Scholar] [CrossRef]
- Jaccob, A.A.; Hussain, S.A.; Hussain, S.A. Effects of long-term use of flavonoids on the absorption and tissue distribution of orally administered doses of trace elements in rats. Pharmacol. Pharm. 2012, 3, 474. [Google Scholar] [CrossRef]
- Coudray, C.; Bousset, C.; Tressol, J.C.; Pépin, D.; Rayssiguier, Y. Short-term ingestion of chlorogenic or caffeic acids decreases zinc but not copper absorption in rats, utilization of stable isotopes and inductively-coupled plasma mass spectrometry technique. Br. J. Nutr. 1998, 80, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Afsana, K.; Shiga, K.; Ishizuka, S.; Hara, H. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Biosci. Biotechnol. Biochem. 2004, 68, 584–592. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol—Rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef]
- Goliomytis, M.; Simitzis, P.; Papalexi, A.; Veneti, N.; Hager-Theodorides, A.L.; Charismiadou, M.A.; Deligeorgis, S.G. Influence of citrus flavonoids on laying hen performance, inflammatory immune response, egg quality and yolk oxidative stability. Br. Poult. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Goliomytis, M.; Orfanou, H.; Petrou, E.; Charismiadou, M.; Simitzis, P.; Deligeorgis, S. Effect of hesperidin dietary supplementation on hen performance, egg quality and yolk oxidative stability. Br. Poult. Sci. 2014, 55, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Zoidis, E.; Georgiou, C.A.; Demiris, N.; Surai, P.F.; Fegeros, K. Influence of organic selenium supplementation on the accumulation of toxic and essential trace elements involved in the antioxidant system of chicken. Food Addit. Contam. 2011, 28, 446–454. [Google Scholar] [Green Version]
- Georgiou, C.A.; Danezis, G.P. Comprehensive Analytical Chemistry; Pico, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, Chapter 3; pp. 131–243. [Google Scholar]
- Richards, M.P. Trace mineral metabolism in the avian embryo. Poult. Sci. 1997, 76, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.D. Trace minerals and avian embryo development. Ciênc. Anim. Bras. 2000, 2, 1–10. [Google Scholar]
- Siddique, S.; Firdous, S.; Durrani, A.I.; Khan, S.J.; Saeed, A. Hesperidin, a citrus flavonoid, increases the bioavailability of micronutrients of Gallus domesticus (chicken) eggshell: In Vitro study. Chem. Speciation Bioavailability 2016, 28, 88–94. [Google Scholar]
- Hashimoto, A.; Kambe, T. Mg, Zn and Cu transport proteins: A brief overview from physiological and molecular perspectives. J. Nutr. Sci. Vitaminol. 2015, 61, 116–118. [Google Scholar]
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ganapathy, M.E.; Leibach, F.H. Intestinal transport of peptides and amino acids. Curr. Top. Membr. 2000, 50, 379–412. [Google Scholar]
- Roy, A.S.; Ghosh, P. Characterization of the binding of flavanone hesperetin with chicken egg lysozyme using spectroscopic techniques: Effect of pH on the binding. J. Inclusion Phenom. Macrocycl. Chem. 2016, 84, 21–34. [Google Scholar]
- Nakamura, S.; Kato, A.; Kobayashi, K. Enhanced antioxidative effect of ovalbumin due to covalent binding of polysaccharides. J. Agric. Food Chem. 1992, 40, 2033–2037. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Hoq, M.I.; Aoki, T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Biol. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; El Mohsen, M.M.A.; Rice-Evans, C. Cellular uptake and metabolism of flavonoids and their metabolites: Implications for their bioactivity. Arch. Biochem. Biophys. 2004, 423, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Piskula, M.K.; Terao, J. Accumulation of (−)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J. Nutr. 1998, 128, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Colotti, G.; Ilari, A.; Boffi, A.; Morea, V. Metals and metal derivatives in medicine. Mini-Rev. Med. Chem. 2013, 13, 211–221. [Google Scholar] [PubMed]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Hameed, S. Radical scavenging propensity of Cu2+, Fe3+ complexes of flavonoids and in-vivo radical scavenging by Fe3+-primuletin. Spectrochim. Acta Part A 2017, 171, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Meyer zu Vilsendorf, A.; Bartels, M.; Pichlmayr, R. Antioxidative vitamins in prevention of ischemia/reperfusion injury. Int. J. Vitam. Nutr. Res. 1997, 67, 298–306. [Google Scholar]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005, 293, 1338–1347. [Google Scholar]
- Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Horcajada, M.N.; Offord-Cavin, E.; Peacock, M.; Weaver, C.M. Effect of hesperidin with and without a calcium (Calcilock) supplement on bone health in postmenopausal women. J. Clin. Endocrinol. Metab. 2016, 101, 923–927. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Saponara, S.; Testai, L.; Iozzi, D.; Martinotti, E.; Martelli, A.; Chericoni, S.; Sgaragli, G.; Fusi, F.; Calderone, V. (+/−)-Naringenin as large conductance Ca2+-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br. J. Pharmacol. 2006, 149, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Fang, X.; Wu, J. Flavonoids function as antioxidants: By scavenging reactive oxygen species or by chelating iron? Radiat. Phys. Chem. 1997, 50, 271–276. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Helena Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; van Balen, G.P.; van den Berg, D.J.; Bast, A.; van der Vijgh, W.J. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin–magnesium complex. Spectrochim. Acta Part A 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, W.; Pang, X.; Wu, W.; Qu, W. Combined effect of total flavonoids from seed residues of Hippophae rhamnoides L. and zinc on advanced glycation end products-induced endothelial cell dysfunction. Food Chem. 2012, 133, 905–911. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Liu, J.; Hong, H.; Yan, C. The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol. Environ. Saf. 2015, 120, 1–6. [Google Scholar] [CrossRef]
- Fisinin, V.I.; Papazyan, T.T.; Surai, P.F. Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit. Rev. Biotechnol. 2009, 29, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Charoensiriwatana, W.; Srijantr, P.; Teeyapant, P.; Wongvilairattana, J. Consuming iodine enriched eggs to solve the iodine deficiency endemic for remote areas in Thailand. Nutr. J. 2010, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.C.; Cheng, K. Selenium enrichment of table eggs. Poult. Sci. 2010, 89, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Aspuru, K.; Villa, C.; Bermejo, F.; Herrero, P.; López, S.G. Optimal management of iron deficiency anemia due to poor dietary intake. Int. J. Gen. Med. 2011, 4, 741–750. [Google Scholar] [PubMed] [Green Version]



| Elements (μg/kg) | Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| C | E1 | E2 | N1 | N2 | VE | SEM | ||
| As | 4.26 | 4.39 | 4.58 | 4.55 | 4.56 | 4.01 | 0.228 | 0.457 |
| Ca | 178,715 b | 170,556 b | 209,284 b | 100,415 a | 95,117 a | 162,672 b | 16,884.5 | <0.001 |
| Cd | 2.45 a | 2.65 a,b | 2.56 a,b | 2.76 b | 3.15 c | 2.41 a | 0.072 | <0.001 |
| Co | 24.95 b | 2.85 a | 2.50 a | 3.46 a | 4.09 a | 8.15 a | 1.642 | <0.001 |
| Cr | 93.78 a | 96.90 a | 94.66 a | 101.48 a,b | 122.85 b | 84.63 a | 5.273 | <0.001 |
| Cu | 326.48 a | 570.76 a,b | 511.90 a,b | 698.76 b | 1241.14 c | 311.84 a | 84.667 | <0.001 |
| Fe | 17,202.9 a,b,c | 18,630 a,b,c | 18,981 b,c | 19,461.3 c | 16,256.1 a | 16,433.4 a,b | 846.16 | 0.037 |
| Mg | 83,029.0 | 95,438.6 | 94,701.7 | 93,771.8 | 84,495.7 | 87,567.8 | 4018.99 | 0.121 |
| Mn | 168.85 b | 111.56 a,b | 104.18 a,b | 82.89 a | 55.41 a | 112.03 a,b | 18.620 | 0.004 |
| Mo | 115.74 | 147.27 | 128.29 | 176.34 | 134.06 | 117.04 | 29.050 | 0.691 |
| Ni | 33.88 a | 78.78 b | 60.31 a,b | 117.51 c | 125.11 c | 33.83 a | 9.150 | <0.001 |
| Pb | 19.21 a | 22.55 a,b | 30.84 b | 25.0 a,b | 25.41 a,b | 16.39 a | 2.682 | 0.009 |
| Sb | 3.19 b | 1.21 a,b | 0.018 a | 0.013 a | 0.033 a | 5.52 c | 0.546 | <0.001 |
| Se | 118.68 a,b | 110.53 a,b | 125.99 b | 99.73 a,b | 91.69 a | 118.98 a,b | 7.538 | 0.022 |
| Sr | 116.84 a | 120.24 a,b | 157.49 b | 115.59 a | 108.05 a | 107.60 a | 8.900 | 0.003 |
| V | 103.46 a | 106.80 a,b | 108.19 a,b | 111.09 a,b | 113.66 b | 101.88 a | 2.261 | 0.005 |
| Zn | 3459.2 a,b | 5262.5 c | 4185.9 a,b,c | 5109.2 b,c | 3306.8 a | 3276.4 a | 551.7 | 0.034 |
| Elements (μg/kg) | Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| C | E1 | E2 | N1 | N2 | VE | SEM | ||
| As | 4.59 | 5.06 | 5.19 | 4.75 | 5.31 | 4.38 | 0.245 | 0.068 |
| Ca | 1.452 × 106 a | 1.728 × 106 b | 1.592 × 106 a,b | 1.653 × 106 b | 1.592 × 106 a,b | 1.402 × 106 a | 47576.6 | <0.001 |
| Cd | 3.06 a | 3.78 b | 3.35 a,b | 3.35 a,b | 3.94 b | 2.90 a | 0.165 | <0.001 |
| Co | 30.44 b | 18.43 a,b | 11.21 a | 7.98 a | 9.85 a | 12.44 a | 3.869 | 0.002 |
| Cr | 144.70 a | 188.11 b | 160.81 a,b | 185.79 b | 192.93 b | 131.88 a | 8.148 | <0.001 |
| Cu | 1485.97 a | 1993.15 b | 1755.32 a,b | 1883.93 a,b | 2444.86 c | 1477.16 a | 105.60 | <0.001 |
| Fe | 76,446.9 a | 93,753.8 b | 83,928.6 a,b | 90,547.8 b | 86,095.7 a,b | 76,851.9 a | 3690.15 | 0.008 |
| Mg | 98,988.3 a | 112,877.1 b | 106,972.2 a,b | 107,764.1 a,b | 102,809.3 a,b | 95,459.3 a | 2675.86 | <0.001 |
| Mn | 1132.21 | 1222.97 | 1093.19 | 1207.33 | 1173.03 | 1193.88 | 103.26 | 0.949 |
| Mo | 843.21 | 1509.76 | 832.99 | 1203.24 | 1360.28 | 735.24 | 255.92 | 0.189 |
| Ni | 56.41 a,b | 104.49 c | 86.83 b,c | 116.43 cd | 145.68 d | 52.91 a | 7.845 | <0.001 |
| Pb | 20.85 a | 38.40 b | 38.21 b | 23.30 a,b | 27.84 a,b | 21.41 a | 4.984 | 0.036 |
| Sb | 2.88 b,c | 1.13 a,b | 0.05 a | 0.013 a | 0.041 a | 4.45 c | 0.483 | <0.001 |
| Se | 457.38 | 520.91 | 466.48 | 483.38 | 472.84 | 466.58 | 19.247 | 0.245 |
| Sr | 534.76 a | 665.54 b | 584.34 a,b | 600.43 a,b | 585.64 a,b | 525.85 a | 28.405 | 0.017 |
| V | 105.29 a,b | 114.51 b,c | 114.33 b,c | 114.33 b,c | 119.26 c | 102.84 a | 2.545 | <0.001 |
| Zn | 43,526.7 a | 55,172.8 b | 48,275.0 a,b | 48,697.4 a,b | 47,815.2 a,b | 44,298.0 a | 2119.24 | 0.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappas, A.C.; Zoidis, E.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Charismiadou, M.A.; Nikitas, C.; Danezis, G.; Deligeorgis, S.G.; Georgiou, C.A. Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study. Antioxidants 2019, 8, 361. https://doi.org/10.3390/antiox8090361
Pappas AC, Zoidis E, Goliomytis M, Simitzis PE, Sotirakoglou K, Charismiadou MA, Nikitas C, Danezis G, Deligeorgis SG, Georgiou CA. Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study. Antioxidants. 2019; 8(9):361. https://doi.org/10.3390/antiox8090361
Chicago/Turabian StylePappas, Athanasios C., Evangelos Zoidis, Michael Goliomytis, Panagiotis E. Simitzis, Kyriaki Sotirakoglou, Maria A. Charismiadou, Christos Nikitas, George Danezis, Stelios G. Deligeorgis, and Constantinos A. Georgiou. 2019. "Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study" Antioxidants 8, no. 9: 361. https://doi.org/10.3390/antiox8090361
APA StylePappas, A. C., Zoidis, E., Goliomytis, M., Simitzis, P. E., Sotirakoglou, K., Charismiadou, M. A., Nikitas, C., Danezis, G., Deligeorgis, S. G., & Georgiou, C. A. (2019). Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study. Antioxidants, 8(9), 361. https://doi.org/10.3390/antiox8090361