A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models
Abstract
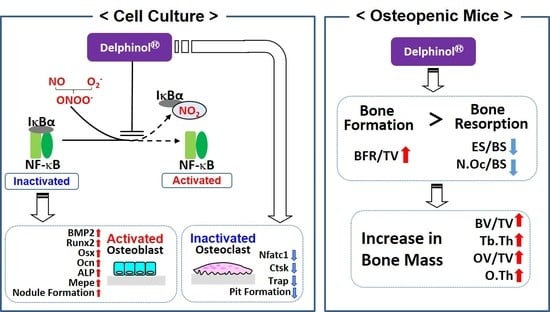
:1. Introduction
2. Materials and Methods
2.1. Maqui Berry Extract
2.2. Effects of MBE on the Differentiation of MC3T3-E1 Cells
2.3. NF-κB Nuclear Translocation and Tyrosine Nitration of I-κB
2.4. OC Differentiation
2.5. Pit Formation
2.6. In Vivo Examination in sRANKL and Ovariectomy-Induced Osteopenic Mouse Models
2.7. Bone Morphometric Analyses
2.8. Statistics
3. Results
3.1. Effects of MBE on OB Differentiation
3.2. Inhibitory Effects of MBE on NF-κB Nuclear Translocation and Nitration of I-κB
3.3. Inhibitory Effects of MBE on OC Differentiation
3.4. Effects of MBE on Pit Formation
3.5. Effects of Administration of MBE on Bone Loss in Osteopenic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Compston, J.; Genant, H. Epidemiology and diagnosis of postmenopausal osteoporosis. In Atlas of Postmenopausal Osteoporosis; Rizzoli, R., Ed.; Springer Healthcare: Tattenhall, UK, 2010; pp. 33–60. [Google Scholar]
- Drake, M.T.; Khosla, S. The role of sex steroids in the pathogenesis of osteoporosis. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Clifford, J., Rosen, M.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 368–370. [Google Scholar]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Invest. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusami, S.; Ramachandran, I.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Jagadeesan, A.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005, 360, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA binding-independent actions of the ERα. J. Bone Miner. Res. 2010, 25, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M. Aging and oxidative stress: A new look at old bone. IBMS BoneKEy 2010, 7, 340–352. [Google Scholar] [CrossRef]
- Khosla, S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Lee, S.; Lee, S.-K.; Chun, O. Dietary Polyphenols, Berries, and Age-Related Bone Loss: A Review Based on Human, Animal, and Cell Studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, H.; Uehara, M.; Wu, J.; Wang, X.; Masuyama, R.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J. Nutr. 2003, 133, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Yamamoto, H.; Sato, T.; Mizuha, Y.; Kawai, Y.; Taketani, Y.; Kato, S.; Terao, J.; Inakuma, T.; Takeda, E. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J. Bone Miner. Metab. 2009, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui [Aristotelia chilensis (Mol.) Stuntz]. Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Vigil, P.P. Delphinol® Standardized Maqui Berry Extract Significantly Lowers Blood Glucose and Improves Blood Lipid Profile in Prediabetic Individuals in Three-Month Clinical Trial. Panminerva Med. 2016, 58, 1–6. [Google Scholar] [PubMed]
- Hitoe, S.; Tanaka, J.; Shimoda, H. MaquiBright® Standardized Maqui Berry Extract Significantly Increases Tear Fluid Production and Ameliorates Dry Eye-Related Symptoms in a Clinical Pilot Trial. Panminerva Med. 2014, 56, 1–6. [Google Scholar]
- Alvarado, J.L.; Leschot, A.; Olivera-Nappa, A.; Salgado, A.M.; Rioseco, H.; Lyon, C.; Vigil, P. Delphinidin-Rich Maqui Berry Extract (Delphinol®) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. Biomed. Res. Int. 2016, 2016, 9070537. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.Y. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.; Strait, K.; Zhang, H.; Nanes, M.S.; Weitzmann, M.N. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J. Bone Miner. Res. 2007, 22, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Krum, S.A.; Chang, J.; Miranda-Carboni, G.; Wang, C.Y. Novel functions for NFκB: Inhibition of bone formation. Nat. Rev. Rheumatol. 2010, 6, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Matata, B.M.; Galiñanes, M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-κB DNA binding activity. J. Biol. Chem. 2001, 277, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shai, M.; Reznick, A.Z. Peroxynitrite induces an alternative NF-kB activation pathway in L8 rat myoblasts. Antioxid. Redox Signal. 2006, 8, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Alund, A.W.; Mercer, K.E.; Pulliam, C.F.; Suva, L.J.; Chen, J.R.; Badger, T.M.; Ronis, M.J. Partial Protection by Dietary Antioxidants Against Ethanol-Induced Osteopenia and Changes in Bone Morphology in Female Mice. Alcohol. Clin. Exp. Res. 2016, 41, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Maeda, T.; Kawane, T.; Horiuchi, N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology 2003, 144, 681–692. [Google Scholar] [CrossRef]
- Tomimori, Y.; Mori, K.; Koide, M.; Nakamichi, Y.; Ninomiya, T.; Udagawa, N.; Yasuda, H. Evaluation of Pharmaceuticals With a Novel 50-Hour Animal Model of Bone Loss. J. Bone Miner. Res. 2009, 24, 1194–1205. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable Concentrations of Delphinidin and Its Metabolite, Gallic Acid, Induce Antioxidant Protection Associated with Increased Intracellular Glutathione in Cultured Endothelial Cells. Oxidative Medicine and Cellular Longevity 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables ; A Review. Front. Chem. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J. Cell Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef]
- Gochman, E.; Mahajna, J.; Reznick, A.Z. NF-κB activation by peroxynitrite through IκBα-dependent phosphorylation versus nitration in colon cancer cells. Anticancer Res. 2011, 31, 1607–1617. [Google Scholar] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Altindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2007, 28, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Sandhu, A.; Edirisinghe, I. Anthocyanins. In Nutraceuticals1st Edition, Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 489–500. [Google Scholar]







| Genes | Proteins (Abbreviations) | Primer Sequences | Product (bp) | Accession Numbers | |
|---|---|---|---|---|---|
| Bmp2 | Bone morphogenetic protein 2 (BMP2) | Forward: | 5′-TGA CTG GAT CGT GGC ACC TC-3′ | 112 | NM_007553.3 |
| Reverse: | 5′-CAG AGT CTG CAC TAT GGC ATG GTT A-3′ | ||||
| Bmp4 | Bone morphogenetic protein 4 (BMP4) | Forward: | 5′-AGC CGA GCC AAC ACT GTG AG-3′ | 68 | NM_007554.2 |
| Reverse: | 5′-TCA CTG GTC CCT GGG ATG TTC-3′ | ||||
| Runx2 | Runt-related transcription factor 2 (RUNX2) | Forward: | 5′-TCA CTA CCA GCC ACC GAG A-3′ | 81 | NM_001145920.2 |
| Reverse: | 5′-CTG CTT GCA GCC TTA AAT ATT CC-3′ | ||||
| Osx | Osterix (OSX) | Forward: | 5′-GTC CTC TCT GCT TGA GGA AGA A-3′ | 79 | NM_130458.4 |
| Reverse: | 5′- GCC AAA TTT GCT GCA GGC T-3′ | ||||
| Mepe | Matrix extracellular phosphoglycoprotein (MEPE) | Forward: | 5′-ATG CAG GGA GAG CTG GTT AC-3′ | 84 | NM_053172.2 |
| Reverse: | 5′-TGG TTC CCT TTG GAC TCT TC-3′ | ||||
| Ocn | Osteocalcin (OCN) | Forward: | 5′-GTG AGC TTA ACC CTG CTT GT-3′ | 96 | NM_130458.4 |
| Reverse: | 5′-AGC ACA GGT CCT AAA TAG TGA TAC C-3′ | ||||
| Actb | β-actin | Forward: | 5′-CAT CCG TAA AGA CCT CTA TGC CAA C-3′ | 171 | NM_007393.5 |
| Reverse: | 5′-ATG GAG CCA CCG ATC CAC A-3′ | ||||
| Nfatc1 | Nuclear factor of activated T cells 1 (NFATC1/NFAT2) | Forward: | 5′-GCC TCG AAC CCT ATC GAG TG-3′ | 121 | AI449492 |
| Reverse: | 5′- AGT TAT GGC CAG ACA GCA CC-3′ | ||||
| Ctsk | Cathepsin K (CTSK) | Forward: | 5′-TAC CCA TAT GTG GGC CAG GA-3′ | 107 | AI323530 |
| Reverse: | 5′- AGT TAT GGC CAG ACA GCA CC-3′ | ||||
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Forward: | 5′- AAC TTT GGC ATT GTG GAA GG -3′ | 132 | NM_008084 |
| Reverse: | 5′- GGA TGC AGG GAT GAT GTT CT -3′ | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaoka, M.; Maeda, T.; Chatani, M.; Handa, K.; Yamakawa, T.; Kiyohara, S.; Negishi-Koga, T.; Kato, Y.; Takami, M.; Niida, S.; et al. A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants 2019, 8, 386. https://doi.org/10.3390/antiox8090386
Nagaoka M, Maeda T, Chatani M, Handa K, Yamakawa T, Kiyohara S, Negishi-Koga T, Kato Y, Takami M, Niida S, et al. A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants. 2019; 8(9):386. https://doi.org/10.3390/antiox8090386
Chicago/Turabian StyleNagaoka, Masahiro, Toyonobu Maeda, Masahiro Chatani, Kazuaki Handa, Tomoyuki Yamakawa, Shuichi Kiyohara, Takako Negishi-Koga, Yasumasa Kato, Masamichi Takami, Shumpei Niida, and et al. 2019. "A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models" Antioxidants 8, no. 9: 386. https://doi.org/10.3390/antiox8090386
APA StyleNagaoka, M., Maeda, T., Chatani, M., Handa, K., Yamakawa, T., Kiyohara, S., Negishi-Koga, T., Kato, Y., Takami, M., Niida, S., Lang, S. C., Kruger, M. C., & Suzuki, K. (2019). A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants, 8(9), 386. https://doi.org/10.3390/antiox8090386