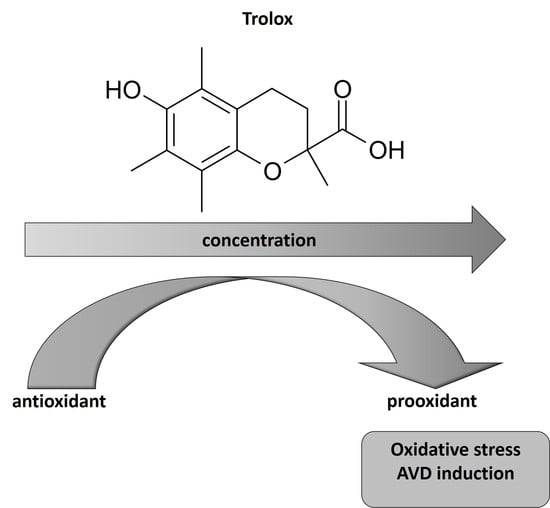
Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Intracellular Oxidative Stress Detection
2.3. Cell Viability Assessment by MTT Assay and Propidium Iodide
2.4. Estimation of Changes in Cell Volume
2.5. Detection of Apoptosis
2.6. Statistics
3. Results
3.1. Dose-Dependent Effect of Trolox on Basal ROS Production and Cell Viability after 24 h Exposure
3.2. Apoptosis Detection
3.3. Dose-Dependent Effect of Trolox on Basal ROS Production and Cell Volume after 2 h Exposure
3.4. Effect of SITS on the Trolox-Induced AVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Massey, K.D.; Burton, K.P. Free radical damage in neonatal rat cardiac myocyte cultures: Effects of alpha-tocopherol, Trolox and phytol. Free Radic. Biol. Med. 1990, 8, 449. [Google Scholar] [CrossRef]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant Activity of Vitamin E and Trolox: Understanding of the factors that govern lipid peroxidation studies in vitro. Food Biophys. 2009, 4, 312–320. [Google Scholar] [CrossRef]
- Birgelius-Flohe, R.; Traber, M.G. Vitamin E Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Distelmaier, F.; Valsecchi, F.; Forkink, M.; van Emst-de Vries, S.; Swarts, H.G.; Rodenburg, R.J.T.; Verwiel, E.T.P.; Smeitink, J.A.M. Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation. Antioxid. Redox. Sign. 2012, 17, 1652–1669. [Google Scholar]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and Ascorbic Acid Reduce Direct and Indirect Oxidative Stress in the IPEC-J2 Cells, an In Vitro Model for the Porcine Gastrointestinal Tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgo, M.G.; Pryor, W.A. Trolox inhibits peroxynitrite-mediated oxidative stress and apoptosis in rat thymocytes. Arch. Biochem. Biophys. 1996, 333, 482–488. [Google Scholar] [CrossRef]
- Messier, E.M.; Bahmed, K.; Tuder, R.M.; Chu, H.W.; Bowler, R.P.; Kosmider, B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013, 4, e573. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Rao, V.R.; Stubbs, E.B.; Kajga, S. Antioxidants protect against reactive astrocytosis-induced sensitization to oxidative stress. Invest. Ophth Vis. Sci. 2019, 60, 3791. [Google Scholar]
- Caro, A.A.; Thompson, S.; Tackett, J. Increased oxidative stress and cytotoxicity by hydrogen sulfide in HepG2 cells overexpressing cytochrome P450 2E1. Cell Biol. Toxicol. 2011, 27, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.H.; Jeong, H.J.; Kim, H.; Leem, Y.E.; Ryu, D.; Park, S.C.; Lee, Y.I.; Cho, S.C.; Kan, J.S. ZNF746/PARIS overexpression induces cellular senescence through FoxO1/p21 axis activation in myoblasts. Cell Death Dis. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, G.D.; Redmond, R.W. Secondary reactive oxygen species extend the range of photosensitization effects in cells: DNA damage produced via initial membrane photosensitization. Photochem. Photobiol. 2003, 77, 192–203. [Google Scholar] [CrossRef]
- Besselink, G.A.; van Engelenburg, F.A.; Ebbing, I.G.; Hilarius, P.M.; de Korte, D.; Verhoeven, A.J. Additive effects of dipyridamole and Trolox in protecting human red cells during photodynamic treatment. Vox Sang. 2003, 85, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Forrest, V.J.; David, Y.H.; McClain, E.; Robinson, D.H.; Ramakrishnan, N. Oxidative stress-induced apoptosis prevented by trolox. Free Radic. Biol. Med. 1994, 16, 675–684. [Google Scholar] [CrossRef]
- Guo, C.; He, Z.; Wen, L.; Zhu, L.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Yuan, H. Cytoprotective effect of trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biol. Int. 2012, 36, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Davargaon, R.S.; Sambe, A.D.; Muthangi, S. Trolox prevents high glucose-induced apoptosis in rat myocardial H9c2 cells by regulating GLUT-4 and antioxidant defense mechanism. IUBMB Life 2019, 71, 1876–1895. [Google Scholar] [CrossRef]
- Xiao, T.; Choudhary, S.; Zhang, W.; Ansari, N.H.; Salahudeen, A. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J. Toxicol. Environ. Health A 2003, 66, 469–479. [Google Scholar] [CrossRef]
- Singh, P.P.; Chandra, A.; Mahdi, F.; Ray, A.; Sharma, P. Reconvene and reconnect the antioxidant hypothesis in Human health and disease. Ind. J. Clin. Biochem. 2010, 25, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive investigation of the antioxidant and pro-oxidant effects of phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. 2018, 122, 6198–6214. [Google Scholar] [CrossRef]
- Albertini, R.; Abuja, P.M. Prooxidant and antioxidant properties of Trolox C, analog of vitamin E, in oxidation of low-density lipoprotein. Free Radic. Res. 1999, 30, 181–188. [Google Scholar] [CrossRef]
- Sharma, M.K.; Buettner, G.R. Interaction of vitamin C and vitamin E during free radical stress in plasma: An ESR study. Free Radic. Biol. Med. 1993, 14, 649–653. [Google Scholar] [CrossRef]
- Ingold, K.U.; Bowry, V.W.; Stocker, R.; Walling, C. Autoxidation of lipids and antioxidation by alpha-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: Unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Raspor, P. The antioxidant and pro-oxidant activity of vitamin C and trolox in vitro: A comparative study. Appl. Toxicol. 2008, 28, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gyulkhandanyan, A.V.; Feeney, C.J.; Pennefather, P.S. Modulation of mitochondrial membrane potential and reactive oxygen species production by copper in astrocytes. J. Neurochem. 2003, 87, 448–460. [Google Scholar] [CrossRef]
- Ko, K.M.; Yick, P.K.; Poon, M.K.T.; Ip, S.P. Prooxidant and antioxidant effects of trolox on ferric ion-induced oxidation of erythrocyte membrane lipids. Mol. Cell Biochem. 1994, 141, 65–70. [Google Scholar] [CrossRef]
- Stewart, M.S.; Spallholz, J.E.; Neldner, K.H.; Pence, B.C. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic. Biol. Med. 1999, 26, 42–48. [Google Scholar] [CrossRef]
- Diaz, Z.; Colombo, M.; Mann, K.K.; Su, H.; Smith, K.N.; Scott Bohle, D.; Schipper, H.M.; Miller, W.H., Jr. Trolox selectively enhances arsenic-mediated oxidative stress and apoptosis in APL and other malignant cell lines. Bood 2005, 105, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Payne, K.; Jori, E.; Taggart, H.J.; Stuart, E.; Ding, L.W.Q. Trolox Enhances Curcumin’s Cytotoxicity through Induction of Oxidative Stress. Cell Physiol. Biochem. 2012, 29, 353–360. [Google Scholar] [CrossRef]
- Bowry, V.W.; Stocker, R. Tocopherol-mediated peroxidation. The pro-oxidant effect of vitamin E on the radicalinitiated oxidation of human low-density lioprotein. J. Am. Chem. Soc. 1993, 115, 6029–6044. [Google Scholar] [CrossRef]
- Tafazoli, S.; Wright, J.S.; O’Brien, P.J. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem. Res. Toxicol. 2005, 18, 1567–1574. [Google Scholar] [CrossRef]
- Maeno, E.; Ishizaki, Y.; Kanaseki, T.; Hazama, A.; Okada, Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9487–9492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lionetto, M.G.; Giordano, M.E.; Calisi, A.; Caricato, R.; Hoffmann, E.K.; Schettino, T. Role of BK channels in the Apoptotic Volume Decrease in native eel intestinal cells. Cell Physiol. Biochem. 2010, 25, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Antico, S.; Lionetto, M.G.; Giordano, M.E.; Caricato, R.; Schettino, T. Cell Volume Regulation and Apoptotic Volume Decrease in rat distal colon superficial enterocytes. Cell Physiol. Biochem. 2013, 32, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Burg, E.D.; Remillard, C.V.; Yuan, J.X.J. K+ channels in apoptosis. J. Membr. Biol. 2006, 209, 3–20. [Google Scholar] [CrossRef]
- Poulsen, K.A.; Andersen, E.C.; Klausen, T.K.; Hougaard, C.; Lambert, I.H.; Hoffmann, E.K. Deregulation of Apoptotic volume decrease and ionic movements in Multidrug Resistant Tumour cells: Role of the chloride permeabililty. Am. J. Physiol. 2009, 298, C14–C25. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002, 9, 1307–1310. [Google Scholar] [CrossRef]
- Maeno, E.; Tsubata, T.; Okada, Y. Apoptotic Volume Decrease (AVD) is independent of mitochondrial dysfunction and initiator caspase activation. Cells 2012, 1, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Latronico, S.; Giordano, M.E.; Urso, E.; Lionetto, M.G.; Schettino, T. Effect of the flame retardant Tris (1,3-dichloro-2-propyl) Phosphate (TDCPP) on Na+-K+-ATPase and Cl- transport in HeLa cells. Toxicol. Mech. Method 2018, 28, 599–606. [Google Scholar] [CrossRef]
- Wattamwar, P.P.; Hardas, S.S.; Butterfield, D.A.; Anderson, K.W.; Dziubla, T.D. Tuning of the pro-oxidant and antioxidant activity of trolox through the controlled release from biodegradable poly(trolox ester) polymers. J. Biomed. Mater. Res. Part A 2011, 99, 184–191. [Google Scholar] [CrossRef]
- O’Gara, B.A.; Murray, P.M.; Hoyt, E.M.; Leigh-Logan, T.; Smeaton, M.B. The Vitamin E analog Trolox reduces copper toxicity in the annelid Lumbriculus variegatus but is also toxic on its own. Neurotoxicology 2006, 27, 604–614. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Cucina, A.; Bizzarri, M.; Mariggiò, M.A. Antioxidant Strategy to Prevent Simulated Microgravity-Induced Effects on Bone Osteoblasts. Int. J. Mol. Sci. 2020, 21, 3638. [Google Scholar] [CrossRef]
- Leventis, P.A.; Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Phelps, P.C.; Berezesky, I.K.; Ebersberger, M.L.; Trump, B.F., Jr. Studies on the Mechanisms and Kinetics of Apoptosis Induced by Microinjection of Cytochrome c in Rat Kidney Tubule Epithelial Cells (NRK-52E). Am. J. Pathol. 2000, 156, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.P.; Choi, D.W. Ions, cell volume, and apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9360–9362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubun, S.; Saigusa, A.; Tamura, T. Blockade of Cl channels by organic and inorganic blockers in vascular smooth muscle cells. Pflfigers Arch. 1991, 418, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 8416763. [Google Scholar] [CrossRef]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2099. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Shimizu, T.; Takahashi, N.; Okada, Y. The apoptotic volume decrease is an upstream event of MAP kinase activation during staurosporine-induced apoptosis in HeLa cells. Int. J. Mol. Sci. 2012, 13, 9363–9379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anglemont de Tassigny, A.; Souktani, R.; Henry, P.; Ghaleh, B.; Berdeaux, A. Volume-sensitive chloride channels (ICl, vol) mediate doxorubicin-induced apoptosis through apoptotic volume decrease in cardiomyocytes. Fundam. Clin. Pharm. 2004, 18, 531–538. [Google Scholar] [CrossRef]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J. Cell. Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Numata, T.; Okada, Y. A role of reactive oxygen species in apoptotic activation of volume sensitive Cl(-) channel. Proc. Natl. Acad. Sci. USA 2004, 101, 6770–6773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato-Numata, K.; Numata, T.; Inoue, R.; Okada, Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflug. Arch. 2016, 468, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Arreola, J.; Melvin, J.E.; Begenisich, T. Volume-activated chloride channels in rat parotid acinar cells. J. Physiol. 1995, 484, 677–687. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease. Antioxidants 2020, 9, 1058. https://doi.org/10.3390/antiox9111058
Giordano ME, Caricato R, Lionetto MG. Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease. Antioxidants. 2020; 9(11):1058. https://doi.org/10.3390/antiox9111058
Chicago/Turabian StyleGiordano, Maria Elena, Roberto Caricato, and Maria Giulia Lionetto. 2020. "Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease" Antioxidants 9, no. 11: 1058. https://doi.org/10.3390/antiox9111058
APA StyleGiordano, M. E., Caricato, R., & Lionetto, M. G. (2020). Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease. Antioxidants, 9(11), 1058. https://doi.org/10.3390/antiox9111058