Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review
Abstract
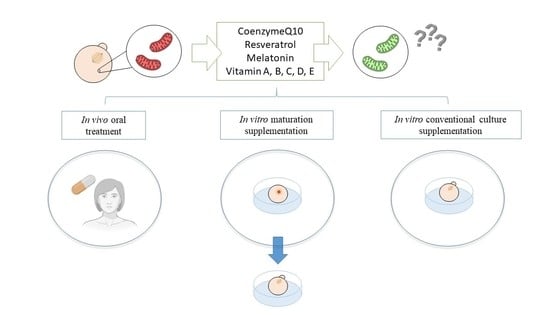
:1. Introduction
2. Antioxidant Supplementation in Reproduction
2.1. Coenzyme-Q10
Use of Coenzyme-Q10 in Infertility
2.2. Resveratrol
Use of Resveratrol in Infertility
2.3. Melatonin
Use of Melatonin in Infertility
2.4. Vitamins
2.4.1. Vitamin A
Use of Vitamin A in Infertility
2.4.2. Folic Acid
Use of Folic Acid in Infertility
2.4.3. Ascorbic Acid
Use of Ascorbic Acid in Infertility
2.4.4. Vitamin D
2.4.5. Vitamin E
Use of Vitamin E in Infertility
2.5. Antioxidants in Combination
2.6. Other Antioxidant Mechanisms
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, E.; Davies, K.J.A. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Marcillat, O.; Giulivi, C.; Ernster, L.; Davies, K.J.A. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990, 265, 16330–16336. [Google Scholar]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Passos, J.F.; Saretzki, G.; Von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Di Lisa, F.; Kaludercic, N.; Carpi, A.; Menabò, R.; Giorgio, M. Mitochondria and vascular pathology. Pharmacol. Rep. 2009, 61, 123–130. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; de la Barca, J.M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier1, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-Y.; Wang, D.H.; Zou, X.Y.; Xu, C.M. Mitochondrial functions on oocytes and preimplantation embryos. J. Zhejiang Univ. Sci. B 2009, 10, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Kuhn, C.; Kolben, T.; Ma, Z.; Lin, P.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Early life oxidative stress and long-lasting cardiovascular effects on offspring conceived by assisted reproductive technologies: A review. Int. J. Mol. Sci. 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Tarín, J.J. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol. Hum. Reprod. 1996, 2, 717–724. [Google Scholar] [CrossRef]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Tiosano, D.; Mears, J.A.; Buchner, D.A. Mitochondrial Dysfunction in Primary Ovarian Insufficiency. Endocrinology 2019, 160, 2353–2366. [Google Scholar] [CrossRef]
- Lampiao, F. Free radicals generation in an in vitro fertilization setting and how to minimize them. World J. Obstet. Gynecol. 2012, 1, 29. [Google Scholar] [CrossRef]
- Van Montfoort, A.P.A.; Arts, E.G.J.M.; Wijnandts, L.; Sluijmer, A.; Pelinck, M.-J.; Land, J.A.; Van Echten-Arends, J. Reduced oxygen concentration during human IVF culture improves embryo utilization and cumulative pregnancy rates per cycle. Hum. Reprod. Open 2020, 2020, hoz036. [Google Scholar] [CrossRef] [PubMed]
- Will, M.A.; Clark, N.A.; Swain, J.E. Biological pH buffers in IVF: Help or hindrance to success. J. Assist. Reprod. Genet. 2011, 28, 711–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobo, A.; Bellver, J.; Domingo, J.; Pérez, S.; Crespo, J.; Pellicer, A.; Remohí, J. New options in assisted reproduction technology: The Cryotop method of oocyte vitrification. RBMO 2008, 17, 68–72. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Harper, J.C. IVF culture media: Past, present and future. Hum. Reprod. Update 2015, 21, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Bentov, Y.; Hannam, T.; Jurisicova, A.; Esfandiari, N.; Casper, R.F. Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clin. Med. Insights Reprod. Health 2014, 8, CMRH.S14681. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplementation on IVF–embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Eryilmaz, O.G.; Devran, A.; Sarikaya, E.; Aksakal, F.N.; Mollamahmutoğlu, L.; Cicek, N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J. Assist. Reprod. Genet. 2011, 28, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2017, 2017, 1–154. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Arcaniolo, D.; Favilla, V.; Tiscione, D.; Pisano, F.; Bozzini, G.; Creta, M.; Gentile, G.; Fabris, F.M.; Pavan, N.; Veneziano, I.A.; et al. Is there a place for nutritional supplements in the treatment of idiopathic male infertility? Arch. Ital. di Urol. e Androl. 2014, 86, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Quadros Gomes, B.A.; Bastos Silva, J.P.; Rodrigues Romeiro, C.F.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Santos Mendes, P.F.; Pompeu Varela, E.L.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonins antioxidant and anti-Aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.K.; Billings, F.T.; Claessens, A.J.; Roshanravan, B.; Linke, L.; Sundell, M.B.; Ahmad, S.; Shao, B.; Shen, D.D.; Ikizler, T.A.; et al. Coenzyme Q10 dose-escalation study in hemodialysis patients: Safety, tolerability, and effect on oxidative stress Dialysis and Transplantation. BMC Nephrol. 2015, 16, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Raizner, A.E. Coenzyme Q10. Methodist Debakey Cardiovasc. J. 2019, 15, 185–191. [Google Scholar] [CrossRef]
- James, A.M.; Smith, R.A.J.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.S.; Tang, P.H.; Morrison, J.A.; Miles, L.; Degrauw, T.; Pesce, A.J. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin. Chim. Acta 2004, 347, 139–144. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Boots, C.E.; Boudoures, A.; Zhang, W.; Drury, A.; Moley, K.H. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum. Reprod. 2016, 31, 2090–2097. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Shin, N.; Karthikraj, R.; Barbosa, F.; Kannan, K.; Colaiácovo, M.P. Antioxidant CoQ10 restores fertility by rescuing bisphenol a-induced oxidative DNA damage in the caenorhabditis elegans germline. Genetics 2020, 214, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Özcan, P.; Fıçıcıoğlu, C.; Kizilkale, O.; Yesiladali, M.; Tok, O.E.; Ozkan, F.; Esrefoglu, M. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J. Assist. Reprod. Genet. 2016, 33, 1223–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; ShiYang, X.; Zhang, Y.; Miao, Y.; Chen, Y.; Cui, Z.; Xiong, B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic. Biol. Med. 2019, 143, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.J.; Zhou, W.; Nie, Z.W.; Zhou, D.; Xu, Y.N.; Ock, S.A.; Yan, C.G.; Cui, X.S. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging (Albany NY) 2020, 12, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Ben-Meir, A.; Kim, K.; McQuaid, R.; Esfandiari, N.; Bentov, Y.; Casper, R.F.; Jurisicova, A. Co-enzyme q10 supplementation rescues cumulus cells dysfunction in a maternal aging model. Antioxidants 2019, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Akarsu, S.; Gode, F.; Isik, A.Z.; Dikmen, Z.G.; Tekindal, M.A. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J. Assist. Reprod. Genet. 2017, 34, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Giannubilo, S.R.; Orlando, P.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Ciavattini, A.; Tiano, L. CoQ10 supplementation in patients undergoing IVF-ET: The relationship with follicular fluid content and oocyte maturity. Antioxidants 2018, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Florou, P.; Anagnostis, P.; Theocharis, P.; Chourdakis, M.; Goulis, D.G. Does coenzyme Q10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J. Assist. Reprod. Genet. 2020, 37, 2377–2387. [Google Scholar] [CrossRef]
- El Refaeey, A.; Selem, A.; Badawy, A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod. Biomed. Online 2014, 29, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Abdulhasan, M.K.; Li, Q.; Dai, J.; Abu-Soud, H.M.; Puscheck, E.E.; Rappolee, D.A. CoQ10 increases mitochondrial mass and polarization, ATP and Oct4 potency levels, and bovine oocyte MII during IVM while decreasing AMPK activity and oocyte death. J. Assist. Reprod. Genet. 2017, 34, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Maside, C.; Martinez, C.A.; Cambra, J.M.; Lucas, X.; Martinez, E.A.; Gil, M.A.; Rodriguez-Martinez, H.; Parrilla, I.; Cuello, C. Supplementation with exogenous coenzyme Q10 to media for in vitro maturation and embryo culture fails to promote the developmental competence of porcine embryos. Reprod. Domest. Anim. 2019, 54, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kile, R.; Logsdon, D.M.; Nathanson, C.; McCormick, S.; Schoolcraft, W.B.; Krisher, R.L. Mitochondrial support of embryos from women of advanced maternal age during ART. Fertil. Steril. 2020, 114, e122. [Google Scholar] [CrossRef]
- Ortega, I.; Duleba, A.J. Ovarian actions of resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 86–96. [Google Scholar] [CrossRef]
- Neves, A.R.; Lucio, M.; Lima, J.L.C.; Reis, S. Resveratrol in Medicinal Chemistry: A Critical Review of its Pharmacokinetics, Drug-Delivery, and Membrane Interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.I.; Nori, S.L. Complementary therapy in polycystic ovary syndrome. Transl. Med. @ UniSa 2014, 9, 56–65. [Google Scholar] [CrossRef]
- Kolahdouz Mohammadi, R.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef]
- Ho, Y.; SH Yang, Y.C.; Chin, Y.T.; Chou, S.Y.; Chen, Y.R.; Shih, Y.J.; Whang-Peng, J.; Changou, C.A.; Liu, H.L.; Lin, S.J.; et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food Chem. Toxicol. 2018, 120, 346–355. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [Green Version]
- Di Emidio, G.; Falone, S.; Vitti, M.; D’Alessandro, A.M.; Vento, M.; Di Pietro, C.; Amicarelli, F.; Tatone, C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum. Reprod. 2014, 29, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Luo, L.L.; Xu, J.J.; Zhuang, X.L.; Kong, X.X.; Fu, Y.C. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 2010, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, Y.; Ye, X.; Zeng, M.; Zhao, Q.; Keefe, D.L.; Liu, L. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013, 28, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-κB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Liu, L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 2018, 44, 1431–1438. [Google Scholar] [CrossRef]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin1, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany NY) 2019, 11, 1030. [Google Scholar] [CrossRef]
- Liu, M.J.; Sun, A.G.; Zhao, S.G.; Liu, H.; Ma, S.Y.; Li, M.; Huai, Y.X.; Zhao, H.; Liu, H. Bin Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kawahara-Miki, R.; Kawana, H.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J. Reprod. Dev. 2015, 61, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Zabihi, A.; Shabankareh, H.K.; Hajarian, H.; Foroutanifar, S. Resveratrol addition to in vitro maturation and in vitro culture media enhances developmental competence of sheep embryos. Domest. Anim. Endocrinol. 2019, 68, 25–31. [Google Scholar] [CrossRef]
- Wong, D.H.; Villanueva, J.A.; Cress, A.B.; Duleba, A.J. Effects of resveratrol on proliferation and apoptosis in rat ovarian theca-interstitial cells. Mol. Hum. Reprod. 2010, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Villanueva, J.A.; Wong, D.H.; Cress, A.B.; Sokalska, A.; Stanley, S.D.; Duleba, A.J. Resveratrol reduces steroidogenesis in rat ovarian theca-interstitial cells: The role of inhibition of Akt/ PKB signaling pathway. Endocrinology 2012, 153, 4019–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergenoglu, M.; Yildirim, N.; Yildirim, A.G.S.; Yeniel, O.; Erbas, O.; Yavasoglu, A.; Taskiran, D.; Karadadas, N. Effects of resveratrol on ovarian morphology, plasma anti-mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod. Sci. 2015, 22, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Wong, D.H.; Villanueva, J.A.; Cress, A.B.; Sokalska, A.; Stanley, S.D.; Duleba, A.J. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil. Steril. 2012, 98, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, R.W.; King, A.E.; Critchley, H.O.D. Cytokine control in human endometrium. Reproduction 2001, 121, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Kuroda, K. Preconception resveratrol intake against infertility: Friend or foe? Reprod. Med. Biol. 2020, 19, 107–113. [Google Scholar] [CrossRef]
- Banaszewska, B.; Wrotyńska-Barczyńska, J.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A.J. Effects of Resveratrol on Polycystic Ovary Syndrome: A Double-blind, Randomized, Placebo-controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 4322–4328. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.T.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Gaspar do Amaral, F.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, G.G.A.; Fioruci-Fontanelli, B.A.; Mendes, L.O.; Ferreira Seiva, F.R.; Martinez, M.; Fávaro, W.J.; Domeniconi, R.F.; Pinheiro, P.F.F.; Delazari dos Santos, L.; Martinez, F.E. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliaferri, V.; Romualdi, D.; Scarinci, E.; De Cicco, S.; Di Florio, C.; Immediata, V.; Tropea, A.; Santarsiero, C.M.; Lanzone, A.; Apa, R. Melatonin Treatment May Be Able to Restore Menstrual Cyclicity in Women with PCOS: A Pilot Study. Reprod. Sci. 2018, 25, 269–275. [Google Scholar] [CrossRef]
- Cagnacci, A. Melatonin in relation to physiology in adult humans. J. Pineal Res. 1996, 21, 200–213. [Google Scholar] [CrossRef]
- Zheng, M.; Tong, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Melatonin concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures. Gynecol. Endocrinol. 2018, 34, 446–450. [Google Scholar] [CrossRef]
- Reiter, R.J. The ageing pineal gland and its physiological consequences. BioEssays 1992, 14, 169–175. [Google Scholar] [CrossRef]
- Vakkuri, O.; Kivelä, A.; Leppäluoto, J.; Valtonen, M.; Kauppila, A. Decrease in melatonin precedes follicle-stimulating hormone increase during perimenopause. Eur. J. Endocrinol. 1996, 135, 188–192. [Google Scholar] [CrossRef]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef]
- Takasaki, A.; Nakamura, Y.; Tamura, H.; Shimamura, K.; Morioka, H. Melatonin as a new drug for improving oocyte quality. Reprod. Med. Biol. 2003, 2, 139–144. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Batioǧlu, A.S.; Şahin, U.; Grlek, B.; Öztrk, N.; Ünsal, E. The efficacy of melatonin administration on oocyte quality. Gynecol. Endocrinol. 2012, 28, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; De Souza, A.; De Souza, I.C.C.; Torres, I.L.S.; Da Cunha Filho, J.S.L.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Aversa, S.; Pellegrino, S.; Barberi, I.; Reiter, R.J.; Gitto, E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Neonatal Med. 2012, 25, 207–221. [Google Scholar] [CrossRef]
- Chattoraj, A.; Bhattacharyya, S.; Basu, D.; Bhattacharya, S.; Bhattacharya, S.; Maitra, S.K. Melatonin accelerates maturation inducing hormone (MIH): Induced oocyte maturation in carps. Gen. Comp. Endocrinol. 2005, 140, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Wallace, E.M.; Vollenhoven, B.; Lolatgis, N.; Hope, N.; Wong, M.; Lawrence, M.; Lawrence, A.; Russell, C.; Leong, K.; et al. Melatonin in assisted reproductive technology: A pilot double-blind randomized placebo-controlled clinical trial. Front. Endocrinol. 2018, 9, 545. [Google Scholar] [CrossRef]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Osorio, N.; Kim, I.J.; Wang, H.; Kaya, A.; Memili, E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J. Pineal Res. 2007, 43, 283–288. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, E.A.; Kim, H.J.; Choi, W.Y.; Cho, J.H.; Lee, W.S.; Cha, K.Y.; You Kim, S.; Lee, D.R.; Yoon, T.K. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS. Reprod. Biomed. Online 2013, 26, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Genario, R.; Morello, E.; Bueno, A.A.; Santos, H.O. The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 2019, 147, 104337. [Google Scholar] [CrossRef] [PubMed]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Schweigert, F.J.; Zucker, H. Concentrations of vitamin A, β-carotene and vitamin E in individual bovine follicles of different quality. J. Reprod. Fertil. 1988, 82, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Graves-Hoagland, R.L.; Hoagland, T.A.; Woody, C.O. Effect of β-Carotene and Vitamin A on Progesterone Production by Bovine Luteal Cells. J. Dairy Sci. 1988, 71, 1058–1062. [Google Scholar] [CrossRef]
- Ikeda, S.; Kitagawa, M.; Imai, H.; Yamada, M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J. Reprod. Dev. 2005, 51, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Livera, G.; Rouiller-Fabre, V.; Valla, J.; Habert, R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol. Cell. Endocrinol. 2000, 165, 225–231. [Google Scholar] [CrossRef]
- Liu, K.H.; Dore, J.J.E.; Roberts, M.P.; Krishnan, R.; Hopkins, F.M.; Godkin, J.D. Expression and Cellular Localization of Retinol-Binding Protein Messenger Ribonucleic Acid in Bovine Blastocysts and Extraembryonic Membranes1. Biol. Reprod. 1993, 49, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Whaley, S.L.; Hedgpeth, V.S.; Farin, C.E.; Martus, N.S.; Jayes, F.C.L.; Britt, J.H. Influence of vitamin A injection before mating on oocyte development, follicular hormones, and ovulation in gilts fed high-energy diets. J. Anim. Sci. 2000, 78, 1598–1607. [Google Scholar] [CrossRef]
- Eberhardt, D.M.; Will, W.A.; Godkin, J.D. Retinol administration to superovulated ewes improves in vitro embryonic viability. Biol. Reprod. 1999, 60, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Swelum, A.A.A.; Saadeldin, I.M.; Noreldin, A.E.; Khafaga, A.F.; Al-Mutary, M.G.; Arif, M.; Hussein, E.S.O.S. The usefulness of retinoic acid supplementation during in vitro oocyte maturation for the in vitro embryo production of livestock: A review. Animals 2019, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, C.; Díez, C.; Duque, P.; Prendes, J.M.; Rodríguez, A.; Goyache, F.; Fernández, I.; Facal, N.; Ikeda, S.; Alonso-Montes, C.; et al. Oocytes recovered from cows treated with retinol become unviable as blastocysts produced in vitro. Reproduction 2005, 129, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri, E.; Mahmoudi, R.; Bahadori, M.H.; Amiri, I. The Effect of Retinoic Acid on In vitro Maturation and Fertilization Rate of Mouse Germinal Vesicle Stage Oocytes. Cell J. 2011, 13, 19–24. [Google Scholar] [PubMed]
- Pauli, S.A.; Session, D.R.; Shang, W.; Easley, K.; Wieser, F.; Taylor, R.N.; Pierzchalski, K.; Napoli, J.L.; Kane, M.A.; Sidell, N. Analysis of follicular fluid retinoids in women undergoing in vitro fertilization: Retinoic acid influences embryo quality and is reduced in women with endometriosis. Reprod. Sci. 2013, 20, 1116–1124. [Google Scholar] [CrossRef]
- Best, M.W.; Wu, J.; Pauli, S.A.; Kane, M.A.; Pierzchalski, K.; Session, D.R.; Woods, D.C.; Shang, W.; Taylor, R.N.; Sidell, N. A role for retinoids in human oocyte fertilization: Regulation of connexin 43 by retinoic acid in cumulus granulosa cells. Mol. Hum. Reprod. 2015, 21, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Damdimopoulou, P.; Chiang, C.; Flaws, J.A. Retinoic acid signaling in ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 2019, 87, 32–41. [Google Scholar] [CrossRef]
- Chan, Y.M.; Bailey, R.; O’Connor, D.L. Folate. Adv. Nutr. 2013, 4, 123–125. [Google Scholar] [CrossRef]
- Kurpad, A.V.; Anand, P.; Dwarkanath, P.; Hsu, J.W.; Thomas, T.; Devi, S.; Thomas, A.; Mhaskar, R.; Jahoor, F. Whole body methionine kinetics, transmethylation, transulfuration and remethylation during pregnancy. Clin. Nutr. 2014, 33, 122–129. [Google Scholar] [CrossRef]
- Laanpere, M.; Altmäe, S.; Stavreus-Evers, A.; Nilsson, T.K.; Yngve, A.; Salumets, A. Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viabilityn ure_266 99..113. Nutr. Rev. 2010. [Google Scholar] [CrossRef]
- De Bree, A.; Van Dusseldorp, M.; Brouwer, I.A.; Van Het Hof, K.H.; Steegers-Theunissen, R.P.M. Folate intake in Europe: Recommended, actual and desired intake. Eur. J. Clin. Nutr. 1997, 51, 643–660. [Google Scholar] [CrossRef] [Green Version]
- Jacques, P.F.; Bostom, A.G.; Wilson, P.W.F.; Rich, S.; Rosenberg, I.H.; Selhub, J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am. J. Clin. Nutr. 2001, 73, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edirimanne, V.E.R.; Woo, C.W.H.; Siow, Y.L.; Pierce, G.N.; Xie, J.Y.; Karmin, O. Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can. J. Physiol. Pharmacol. 2007, 85, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Outinen, P.A.; Sood, S.K.; Pfeifer, S.I.; Pamidi, S.; Podor, T.J.; Li, J.; Weitz, J.I.; Austin, R.C. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells—PubMed. Blood 1999, 94, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Willmott, M.; Bartosik, D.B.; Romanoff, E.B. The effect of folic acid on superovulation in the immature rat. J. Endocrinol. 1968, 41, 439–445. [Google Scholar] [CrossRef]
- Mohanty, D.; Das, K.C. Effect of folate deficiency on the reproductive organs of female rhesus monkeys: A cytomorphological and cytokinetic study. J. Nutr. 1982, 112, 1565–1576. [Google Scholar] [CrossRef] [Green Version]
- Thaler, C.J.; Budiman, H.; Ruebsamen, H.; Nagel, D.; Lohse, P. Effects of the common 677C>T mutation of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness to recombinant follicle-stimulating hormone. Am. J. Reprod. Immunol. 2006, 55, 251–258. [Google Scholar] [CrossRef]
- Szymański, W.; Kazdepka-Ziemińska, A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Gynekol. Pol. 2003, 74, 1392–1396. [Google Scholar]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Ionescu-Ittu, R.; Marelli, A.J.; Mackie, A.S.; Pilote, L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: Time trend analysis in Quebec, Canada. BMJ 2009, 338, 1261. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Frías, M.-L.; Pérez, B.; Desviat, L.R.; Castro, M.; Leal, F.; Rodríguez, L.; Mansilla, E.; Martínez-Fernández, M.-L.; Bermejo, E.; Rodríguez-Pinilla, E.; et al. Maternal Polymorphisms 677C-T and 1298A-C of MTHFR, and 66A-G MTRR Genes: Is There Any Relationship Between Polymorphisms of the Folate Pathway, Maternal Homocysteine Levels, and the Risk for Having a Child With Down Syndrome? Am. J. Med. Genet. Part A 2006, 140, 987–997. [Google Scholar] [CrossRef]
- Lindblad, B.; Zaman, S.; Malik, A.; Martin, H.; Ekström, A.M.; Amu, S.; Holmgren, A.; Norman, M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet. Gynecol. Scand. 2005, 84, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Martel, J.; Karahan, G.; Angle, C.; Behan, N.A.; Chan, D.; Macfarlane, A.J.; Trasler, J.M. Moderate maternal folic acid supplementation ameliorates adverse embryonic and epigenetic outcomes associated with assisted reproduction in a mouse model. Hum. Reprod. 2019, 34, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Schutt, A.K.; Blesson, C.S.; Hsu, J.W.; Valdes, C.T.; Gibbons, W.E.; Jahoor, F.; Yallampalli, C. Preovulatory exposure to a protein-restricted diet disrupts amino acid kinetics and alters mitochondrial structure and function in the rat oocyte and is partially rescued by folic acid 06 Biological Sciences 0601 Biochemistry and Cell Biology. Reprod. Biol. Endocrinol. 2019, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Steegers, E.A.P.; Thomas, C.M.G.; Hollanders, H.M.G.; Copius Peereboom-Stegeman, J.H.J.; Trijbels, F.J.M.; Eskes, T.K.A.B. Study on the presence of homocysteine in ovarian follicular fluid. Fertil. Steril. 1993, 60, 1006–1010. [Google Scholar] [CrossRef]
- Brouwer, I.A.; Van Dusseldorp, M.; Thomas, C.M.G.; Duran, M.; Hautvast, J.G.A.J.; Eskes, T.K.A.B.; Steegers-Theunissen, R.P.M. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: A randomized trial. Am. J. Clin. Nutr. 1999, 69, 99–104. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Peters, W.H.M.; Thomas, C.M.G.; Wetzels, A.M.M.; Peer, P.G.M.; Steegers-Theunissen, R.P.M. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum. Reprod. 2006, 21, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Berker, B.; Kaya, C.; Aytac, R.; Satiroglu, H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum. Reprod. 2009, 24, 2293–2302. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Afeiche, M.C.; Wright, D.L.; Toth, T.L.; Williams, P.L.; Gillman, M.W.; Hauser, R.; Chavarro, J.E. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet. Gynecol. 2014, 124, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Elkin, A.C.; Higham, J. Folk acid supplements are more effective than increased dietary folate intake in elevating serum folate levels. BJOG An Int. J. Obstet. Gynaecol. 2000, 107, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Murto, T.; Skoog Svanberg, A.; Yngve, A.; Nilsson, T.K.; Altmäe, S.; Wånggren, K.; Salumets, A.; Stavreus-Evers, A. Folic acid supplementation and IVF pregnancy outcome in women with unexplained infertility. Reprod. Biomed. Online 2014, 28, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Castillo-Martín, M.; Bonet, S.; Morató, R.; Yeste, M. Supplementing culture and vitrification-warming media with L-ascorbic acid enhances survival rates and redox status of IVP porcine blastocysts via induction of GPX1 and SOD1 expression q. Cryobiology 2014, 68, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Nohalez, A.; Martinez, C.A.; Parrilla, I.; Roca, J.; Gil, M.A.; Rodriguez-Martinez, H.; Martinez, E.A.; Cuello, C. Exogenous ascorbic acid enhances vitrification survival of porcine in vitro-developed blastocysts but fails to improve the in vitro embryo production outcomes. Theriogenology 2018, 113, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crha, I.; Hrubá, D.; Ventruba, P.; Fiala, J.; Totušek, J.; Višňová, H. Ascorbic acid and infertility treatment. Cent. Eur. J. Public Health 2003, 11, 63–67. [Google Scholar]
- Griesinger, G.; Franke, K.; Kinast, C.; Kutzelnigg, A.; Riedinger, S.; Kulin, S.; Kaali, S.G.; Feichtinger, W. Ascorbic Acid Supplement During Luteal Phase in IVF. J. Assist. Reprod. Genet. 2002, 19, 164–168. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization–embryo transfer in endometriosis: A randomized controlled study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum. Reprod. 2018, 33, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Paffoni, A.; Somigliana, E.; Sarais, V.; Ferrari, S.; Reschini, M.; Makieva, S.; Papaleo, E.; Viganò, P. Effect of vitamin D supplementation on assisted reproduction technology (ART) outcomes and underlying biological mechanisms: Protocol of a randomized clinical controlled trial. The “supplementation of vitamin D and reproductive outcome” (SUNDRO) study. BMC Pregnancy Childbirth 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Li, X.; Sun, J.; Ding, G.; Zhou, M.; Zhao, W.; Lu, Y. The effects of calcipotriol on the dendritic morphology of human melanocytes under oxidative stress and a possible mechanism: Is it a mitochondrial protector? J. Dermatol. Sci. 2015, 77, 117–124. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.E.; Seidel, G.E. Culture of In Vitro-Produced Bovine Embryos with Vitamin E Improves Development In Vitro and After Transfer to Recipients. Biol. Reprod. 2000, 62, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Falcone, T.; Attaran, M.; Goldberg, J.M.; Agarwal, A.; Sharma, R.K. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil. Steril. 2002, 78, 1272–1277. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Pertusa, J.F.; Cano, A. Oral administration of pharmacological doses of Vitamins C and E reduces reproductive fitness and impairs the ovarian and uterine functions of female mice. Theriogenology 2002, 57, 1539–1550. [Google Scholar] [CrossRef]
- Bahadori, M.H.; Sharami, S.H.; Fakor, F.; Milani, F.; Pourmarzi, D.; Dalil-Heirati, S.F. Level of Vitamin E in Follicular Fluid and Serum and Oocyte Morphology and Embryo Quality in Patients Undergoing IVF Treatment. J. Fam. Reprod. Health 2017, 11, 74. [Google Scholar]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Xian, Y.; Liang, L.; Qi, S.; Xie, Y.; Song, B.; Ouyang, S.; Xie, Y.; Sun, X.; Wang, W. Antioxidants retard the ageing of mouse oocytes. Mol. Med. Rep. 2018, 18, 1981–1986. [Google Scholar] [CrossRef]
- Silva, E.; Greene, A.F.; Strauss, K.; Herrick, J.R.; Schoolcraft, W.B.; Krisher, R.L. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reprod. Fertil. Dev. 2015, 27, 975–983. [Google Scholar] [CrossRef]
- Youssef, M.A.F.M.; Abdelmoty, H.I.; Elashmwi, H.A.; Abduljawad, E.M.; Elghamary, N.; Magdy, A.; Mohesen, M.N.; Abdella, R.M.A.; Bar, M.A.; Gouda, H.M.; et al. Oral antioxidants supplementation for women with unexplained infertility undergoing ICSI/IVF: Randomized controlled trial. Hum. Fertil. 2014, 18, 38–42. [Google Scholar] [CrossRef]
- Özkaya, M.O.; Nazıroğlu, M.; Barak, C.; Berkkanoglu, M. Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol. Trace Elem. Res. 2011, 139, 1–9. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.Y.; Wang, X.; Yu, Q.; Li, H.Y.; Li, S.J.; Tang, R.Y.; Guo, Z.X.; Chen, Y.Q.; Hu, C.X.; Yang, Z.J.; et al. Evidence that growth hormone can improve mitochondrial function in oocytes from aged mice. Reproduction 2019, 153, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Güleç Başer, B.; Taşkın, M.İ.; Adalı, E.; Öztürk, E.; Hısmıoğulları, A.A.; Yay, A. Does progesterone have protective effects on ovarian ischemia-reperfusion injury? J. Turkish-German Gynecol. Assoc. 2018, 19, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Eser, A.; Hizli, D.; Namuslu, M.; Haltas, H.; Kosus, N.; Kosus, A.; Kafali, H. Protective effect of curcumin on ovarian reserve in a rat ischemia model: An experimental study. Clin. Exp. Obstet. Gynecol. 2017, 44, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mo, G.; Tao, Y.; Wang, H.; Johné Liu, X. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod. Fertil. Dev. 2017, 7, 1392–1400. [Google Scholar] [CrossRef]
- Xu, W.; Li, L.; Sun, J.; Zhu, S.; Yan, Z.; Gao, L.; Gao, C.; Cui, Y.; Mao, C. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging (Albany NY) 2018, 10, 4093. [Google Scholar] [CrossRef]
- Tao, Y.; Tartia, A.; Lawson, M.; Zelinski, M.B.; Wu, W.; Liu, J.Y.; Smitz, J.; Léveillé, M.C.; Leader, A.; Wang, H.; et al. Can peri-ovulatory putrescine supplementation improve egg quality in older infertile women? J. Assist. Reprod. Genet. 2019, 36, 395–402. [Google Scholar] [CrossRef]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The Effect of Retarded Growth Upon the Length of Life Span and Upon the Ultimate Body Size. J. Nutr. 1935, 10, 63–79. [Google Scholar] [CrossRef]
- Selesniemi, K.; Lee, H.J.; Tilly, J.L. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008, 7, 622–629. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Du, D.; Wu, T.; Wen, J.; Wu, M.; Zhang, Y.; Yan, W.; Zhou, S.; Li, Y.; et al. Can ovarian aging be delayed by pharmacological strategies? Aging (Albany NY) 2019, 11, 817. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Huang, X.; Xu, B.; Yan, Y.; Zhang, Q.; Li, Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Lin, D.; Zhang, Y.; Lin, Y.; Song, J.; Li, S.; Sun, Y. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine 2017, 96, e8842. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, L.; Stracquadanio, M.; Pagano, I.; Carbonaro, A.; Palumbo, M.; Gulino, F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: A double blind trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 509–514. [Google Scholar] [PubMed]
- Unfer, V.; Raffone, E.; Rizzo, P.; Buffo, S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011, 27, 857–861. [Google Scholar] [CrossRef] [PubMed]




| Antioxidant | Authors | Type of Study | In Vitro/In Vivo | Intervention | Primary Endpoint | Condition | Treatment Groups (n) | Findings |
|---|---|---|---|---|---|---|---|---|
| CoQ10 | Bentov et al., 2014 [26] | RCT | In Vivo | 600 mg/day for 2 months until the day of oocyte retrieval. Double blinded. | Number of euploid embryos per retrieval | IVF patients 35–43 y.o. | Study gr.: 17 Control gr: 22 | Lower aneuploidy rate in the CoQ10 group (46.5% vs. 62.8% in the control group; p = NS). Premature termination of the study. |
| El Refaeey et al., 2014 [50] | RCT | In Vivo | 60 mg three times per day from day 2 of cycle until the day of ovulation induction. Blinded. | Number of follicles >14 and ≥18 mm | CC-resistant PCOS | Study gr.: 51 Control gr.: 50 | In the treatment group, higher number of follicles >14 mm (1.94 ± 0.25 vs. 0.13 ± 0.29; p < 0.05) and of follicles ≥18 mm (1.85 ± 0.27 vs. 1.30 ± 0.32; p < 0.001). | |
| Xu et al., 2018 [48] | RCT | In Vivo | 600 mg/day for 60 days before the initiation of ovarian stimulation | Number of high-quality day-3 embryos | Poor ovarian response patients <35 y.o. | Study gr.: 76 Control gr.: 93 | Higher mean number of good quality day-3 embryos in the CoQ10 group (1 vs. 0 in the control group; p = 0.03). Secondary endpoints: - Higher ovarian response in the CoQ10 group (4 vs. 2 mean retrieved oocytes; p = 0.002). - Higher fertilization rate in the CoQ10 treatment group (67.5% vs. 45.1%; p = 0.001). - No significant differences in CPR, MR, and LBR. | |
| Ma et al., 2020 [53] | RCT | In Vitro | IVM medium supplemented with 50 mmol/L for 24 h | Oocyte maturation and postmeiotic aneuploidy rates | IVM of GV from patients ≥38 y.o. and patients ≤30 y.o. | 45 patients ≥38 y.o. Study gr.: 46GV Control gr.: 46GV18 patients ≤30 y.o. Study gr.: 35GV Control gr.: 39GV | Patients ≥38 y.o.: - Higher maturation rate in the CoQ10 group (82.6% vs. 63.0%; p = 0.035). - Reduced postmeiotic aneuploidy rate in the CoQ10 group (36.8% vs. 65.5%; p = 0.02). Patients ≤30 y.o.: - Similar maturation rates (80.0% in the CoQ10 group vs. 76.9% in the control group; p = 0.8). - Similar postmeiotic aneuploidy rates (28.6% in the CoQ10 group vs. 30.0% in the control group; p = 0.9). | |
| CoQ10 | Kile et al., 2020 [54] | RCT Preliminary results (ASRM Congress 2020) | In Vitro | Mitoquinol addition to the culture media from fertilization and throughout embryo development | Effect on embryo development | Advanced maternal age (≥35 y.o.) women | 11 patients Study gr: 66 embryos Control gr: 143 embryos | No differences between control and Mitoquinol treatment in day 5 (18% in control group vs. 20% in the study group) or total (48% vs. 45%) good quality blastocyst development per zygote, total blastocyst development (63% vs. 62%) and euploidy rates (33% vs. 30%); p = NS. |
| Resveratrol | Liu et al., 2018 [68] | RCT | In Vitro | IVM medium supplemented with 1.0 µm for 24 and 36 h | Maturation rates after 24 and 36 h, mitochondrial immunofluores-cence intensity, and proportion of matured oocytes with an abnormal spindle morphology and irregular chromosomal arrangement | IVM of GV from patients 38–45 y.o. | 64 patients Study gr.: 38GV Control gr.: 37GV | - Increased maturation rates of the resveratrol group after 24 h (55.3% vs. 37.84% in the control group; p < 0.05) and 36 h (71.1% vs. 51.35%; p < 0.05) of IVM culture. - Increased mitochondrial immunofluorescence intensity in the resveratrol group (53.0% vs. 31.1%, p < 0.05). - Reduced proportion of abnormal spindle morphology and irregular chromosomal arrangement in the resveratrol group (p < 0.05). |
| Bahramrezaie et al., 2019 [78] | RCT | In Vivo | 800 mg/day for 40 days until the day of oocyte retrieval. Triple blinded. | Levels of VEGF expression in granulosa cells | Infertile PCOS patients 18–40 y.o. | Study gr.: 30 Control gr.: 31 | - Reduced VEGF expression in the resveratrol group (p = 0.0001). Secondary endpoints: - No differences between both groups in the number of mature oocytes and cleavage and fertilization rates (p = NS). - Higher high-quality oocyte rate (81.9% vs. 69.1%; p = 0.002) and high-quality embryo rate in the resveratrol group (89.8% vs. 78.8%; p = 0.024). | |
| Resveratrol | Ochiai et al., 2019 [27] | Retrospective | In Vivo | 200 mg/day during the IVF cycle. | Pregnancy outcomes (CPR and MR) | IVF patients | Study gr.: 204 cycles/102 women Control gr.: 7073 cycles/2958 women | Decreased CPR [10.8% vs. 21.5%; p = 0.0005 (Adjusted OR 95% CI 0.539, 0.341–0.853] and increased MR [52.4% vs. 21.8%; p = 0.0022 (Adjusted OR 95% CI 2.602, 1.070–6.325] after resveratrol supplementation. |
| Melatonin | Takasaki et al., 2003 [90] | Prospective cohort study with an intrapatient retrospective comparison | In Vivo | 1 or 3 mg/day from the fifth day of the previous cycle until the day of ovulation induction | To compare oocyte quality between the previous and current IVF cycles | Women with a previous IVF failure due to poor oocyte quality | Study gr. (1 mg): 13 Control gr.: previous cycle data. Study gr. (3 mg): 23 Control gr.: previous cycle data. | - Reduced number of degenerated oocytes in the 3 mg group (p < 0.05) vs. the control group. - Tendency toward an increased fertilization rate in the 3 mg group. - No differences in the numbers of retrieved and mature oocytes between the 3 mg and the control group. - No differences in the number of retrieved, mature, degenerated, and fertilized oocytes between the 1 mg and control group. |
| Tamura et al., 2008 [91] | Prospective cohort study with a retrospective comparison in the same population | In Vivo | 3 mg/day from the fifth day of the previous cycle until the day of oocyte retrieval | To compare fertilization rates between the previous and current IVF cycles | Women with a previous IVF failure due to low fertilization rate (≤50%) | Study gr.: 56 Control gr.: previous cycle data Placebo gr.: 59 Control gr.: previous cycle data | Increased fertilization rate in the melatonin group (29.8 points compared to the previous IVF cycle; p < 0.01), while there were no differences in the placebo group (1.9 points compared to the previous IVF cycle; p > 0.01). | |
| Eryilmaz et al., 2011 [28] | RCT | In Vivo | 3 mg/day from the third or fifth day of the previous cycle until the day of oocyte retrieval | Oocyte quality | IVF patients | Study gr.: 30 Control gr.: 30 | - Increased number of mature oocytes (9.0 vs. 4.4; p = 0.0001) in the treated group. Secondary endpoints: - Increased number of retrieved oocytes (11.5 vs. 6.9; p = 0.0001) in the treated group. | |
| Batıoğlu et al., 2012 [92] | RCT | In Vivo | 3 mg/day during the IVF cycle | Number of MII oocytes | IVF patients | Study gr.: 40 Control gr.: 45 | - No differences in the mean number of MII oocytes retrieved (12.0 in the study vs. 10.9 in the control gr.; p = 0.139). - Higher percentage of MII oocytes/retrieved in the treated group (81.9% in the study vs. 75.8% in the control gr.; p = 0.034). | |
| Melatonin | Kim et al., 2013 [100] | RCT | In Vitro | IVM medium supplemented with 10 µmol/L for 24 and 48 h | Maturation rates | IVM of GV from PCOS patients with or without hCG priming during unstimula-ted cycles | Study gr.: 62 (41 non-hCG primed, 21 hCG primed) Control gr.: 49 (25 non-hCG primed, 24 hCG primed) | - In the non-hCG priming gr., there were no differences in the maturation rate between melatonin treatment and the control gr. after 24 h (40.0% vs. 40.0%; p = NS) and 48 h (62.5% vs. 60.3%; p = NS) of maturation. - In the hCG priming gr., there were no differences in the maturation rate between melatonin treatment and control gr. after 24 h (51.3% vs. 44.9%; p = NS) and 48 h (59.8% vs. 54.8%; p = NS) of maturation. |
| Fernando et al., 2018 [96] | RCT | In Vivo | 2/4/8 mg/twice per day during ovarian stimulation. Double blind. | CPR | IVF patients | Study gr. (2 mg): 41 Study gr. (4 mg): 39 Study gr. (8 mg): 40 Control gr.: 40 | - No differences in CPR (15% in the control group vs. 26.8 in the 2 mg group; 15.4 in the 4 mg group; 22.5 in the 8 mg group; p = 0.5). Secondary endpoints: - No differences in the total oocyte number (p = 0.8), number of MII oocytes (p = 0.4), number of fertilized oocytes (p = 0.6) and the number (p = 0.6) or quality (p = 0.9) of embryos between any of the three treatment groups and the control group. | |
| Espino et al., 2019 [97] | RCT | In Vivo | 3 or 6 mg/day from the onset of ovarian stimulation until the day of oocyte retrieval | - TAC, SOD, and LPO as antioxidant markers in the follicular fluid. - 8-OHdG as oxidative stress marker in the follicular fluid. - IVF success. | IVF patients with unexplained infertility | Study gr. (placebo): 10 Study gr. (3 mg): 10 Study gr. (6 mg): 10 Non-randomized control gr. (fertile women): 10 | - Restored concentrations of TAC, SOD, LPO, and 8-OHdG in the follicular fluid of the study gr. to levels found in fertile women (except for SOD levels in the 3 mg study gr.). - Improved MII oocyte rate (83.6% in the 3 mg gr. vs. 81.9 in the fertile gr., p = NS, and in comparison to 70.6 in the no-treatment gr., p < 0.05). - Improved MII oocyte rate (76.2% in the 6 mg gr., vs. 81.9% in the fertile gr. and 70.6% in the no-treatment gr.; p = NS). - Increased number of transferable embryos (5.1 in the 3 mg gr. and 4.6 in the 6 mg gr.; vs. 2.3 in the fertile gr., p < 0.05, and 2.0 in the no-treatment gr., p < 0.05). | |
| Folic acid | Gaskins et al., 2014 [138] | Prospective cohort | In Vivo | Validated food frequency questionnaire, with specific data about supplemental folic acid intake | To assess the relationship between pregnancy outcomes (implantation rate, CPR, and LBR) and supplemental folate intake | Infertile IVF patients | Total n: 232 Quartile 1 (Q1) Folic acid <400 µg/day): 51 Quartile 4 (Q4) Folic acid >800 µg/day): 60 | - Higher implantation rates [Adjusted mean; A.m., 95% CI 0.67 (0.56, 0.77) vs. 0.43 (0.31, 0.55)], CPR [A.m., 95% CI 0.62 (0.51, 0.73) vs. 0.41 (0.29, 0.53)], and LBR [A.m., 95% CI 0.55 (0.43, 0.66) vs. 0.35 (0.24, 0.48)], in the Q4 in comparison to Q1 (p < 0.05). - Positive linear relationship between supplemental folate and LBR up to 1200 µg/day, without evidence of additional benefit with higher intakes. |
| Murto et al., 2014 [140] | Longitudinal cohort study | In Vivo | Serum folate determinations (folate status) and folic acid supplement questionnaires (folic acid intake) | CPR and LBR | IVF patients with unexplained infertility | Total n: 180 [Serum folate] ≥22.5 nmol/L: 78/180<22.5 nmol/L: 89/180 No data: 13 Folic acid supplements intake Users: 137/180 Non-users: 42/180 No data: 1/180 | Folate status: - No statistically significant differences regarding CPR [35.9% when serum folate ≥22.5 nmol/L vs. 34.8%; OR 95% CI 0.954 (0.505–1.802)] and LBR [28.2% when serum folate ≥22.5 nmol/L vs. 27.0%; OR 95% CI 0.940 (0.476–1.855)]. Folic acid intake: - No statistically significant differences regarding CPR [32.8% users vs. 35.7% non-users; OR 95% CI 1.003 (0.515–1.953)] and LBR [24.1% users vs. 31.0% non-users; OR 95% CI 1.366 (0.677–2.757)]. | |
| Ascorbic acid | Griesinger et al., 2002 [145] | RCT | In Vivo | 1/5/10 g/day from the day of oocyte retrieval and during the luteal phase (14 days). Double blind. | Implantation rate and CPR | Infertile IVF patients | Study group (1g): 172 Study group (5g): 153 Study group (10g): 136 Control gr.:158 | - Implantation rate was 10.0% in the 1 g group, 12.36% in the 5 g group, 10.3% in the 10 g group, and 14.8% in the control group (p = 0.186). - CPR was 22% in the 1 g group, 24% in the 5 g group, 21% in the 10 g group, and 28% in the control group (p = 0.186). |
| Ascorbic acid | Crha et al., 2003 [144] | Prospective cohort | In Vivo | 500 mg/day during ovarian stimulation | Number of pregnancies | Infertile IVF patients | Study gr.: 38 Control gr.: 38 | No significant difference in the number of pregnancies (34.2% vs. 23.7% in the control group; p = NS). |
| Lu et al., 2018 [146] | RCT | In Vivo | 1000 mg/day from 2 months before IVF treatment until 2 weeks after embryo transfer | - Serum and follicular fluid levels of ascorbic acid. - Levels of oxidative stress markers | Endometriosis patients | Study gr.: 137 Placebo gr.: 108 | - Higher serum and follicular fluid levels of ascorbic acid (levels not shown; p < 0.05) in the study group. - No difference in oxidative stress markers after treatment. | |
| Vitamin E | Bahadori et al., 2017 [155] | Observational | In Vivo | Serum and follicular fluid vitamin E determination | To assess the relationship between serum and follicular fluid vitamin E levels and oocyte maturation and embryo quality | IVF patients with a history of vitamin E supplementa-tion | Total n: 50 Follicular fluid ranges (mg/dL) 0.35–1 1–1.5 1.5–2 2–7.4 Serum ranges (mg/dL) 1-5 5-10 10–15 15–0 | Follicular fluid: - Vitamin E levels within the ranges of 0.35–1 mg/dL and 1.5–2 mg/dL were related to higher oocyte maturation rates (89.2% and 84.9%, respectively, vs. 69.6% in 1–1.5 mg/dL and 76.7% in 2–7.4 mg/dL ranges; p = 0.002). - No significant relationship between vitamin E levels and embryo quality was observed. Serum: - Vitamin E levels between 10 and 15 mg/dL were correlated with a higher proportion of high-quality embryos (87.5% vs. 46.2 in 1-5 mg/dL, 54.9% in 5–10 mg/dL, 42.9% in 15-20 mg/dL; p = 0.007). - No significant relationship between vitamin E levels and oocyte maturation was observed. A higher proportion of MII oocytes in women with vitamin E supplementation (87.4% vs.77% in women without supplementation; p = 0.010). |
| Antioxidants in combination | Fatemi et al., 2017 [156] | RCT | In Vivo | Vitamin E (400 mg/day) and Vitamin D (50,000 IU/one in two weeks) for 8 weeks. Double blinded. | Implantation rate, pregnancy rate, and CPR | PCOS infertile women | Study gr.: 44 Control gr.: 46 | Higher implantation (35.1% vs. 8.6%; p < 0.001), pregnancy (69.0% vs. 25.8%; p < 0.001), and CPR (62.1% vs. 22.6%; p = 0.002) in the treated group. |
| Ozkaya et al., 2011 [160] | RCT | In Vivo | Vitamins A, B, C, D, E, H; calcium; folic acid; iron; nicotinic acid; magnesium; phosphor; copper; manganese; zinc For 45 days before serum and follicular fluid collection | Follicular fluid and serum antioxidant capacity | IVF patients | Study gr.: 26Placebo gr.: 30 | - Higher serum and follicular fluid antioxidant capacity were observed in the treated group. - Higher serum vitamins C (61.6 µmol/L vs. 57.9 µmol/L in the control group; p < 0.05) and A (2.3 µmol/L vs. 1.5 µmol/L; p < 0.01); and higher follicular fluid glutathione (0.4 µmol/L vs. 0.2 µmol/L; p < 0.01) and vitamin C (84.5 µmol/L vs. 52.7 µmol/L; p < 0.01) and E (8.3 µmol/L vs. 5.0 µmol/L; p < 0.001) concentrations. | |
| Youssef et al., 2014 [159] | RCT | In Vivo | Vitamins A, E, C Zinc Molybdenum Selenium Biotin Bioflavonoid | Number of MII oocytes | IVF patients with unexplained infertility | Study group: 112 Control group: 106 | No difference in the mean number of MII oocytes between the treated (12.7) and the control group (13.2); p = 0.7. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Varela, C.; Labarta, E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197. https://doi.org/10.3390/antiox9121197
Rodríguez-Varela C, Labarta E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants. 2020; 9(12):1197. https://doi.org/10.3390/antiox9121197
Chicago/Turabian StyleRodríguez-Varela, Cristina, and Elena Labarta. 2020. "Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review" Antioxidants 9, no. 12: 1197. https://doi.org/10.3390/antiox9121197
APA StyleRodríguez-Varela, C., & Labarta, E. (2020). Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants, 9(12), 1197. https://doi.org/10.3390/antiox9121197