Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Assays
2.3. Statistical Analyses
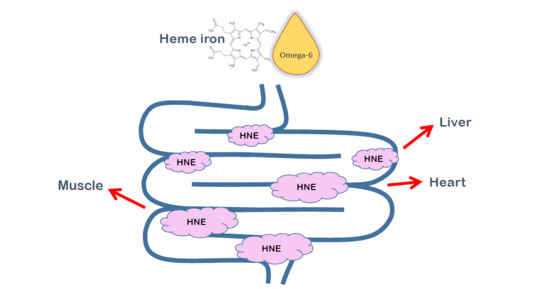
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institut National du Cancer. Nutrition et Prévention des Cancers: Actualisation des Données, Collection Etat des Lieux et des Connaissances; Institut National du Cancer: Boulogne-Billancourt, France, 2015; ISBN 978-2-37219-114-2. (In French) [Google Scholar]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Chenni, F.; Audebert, M.; Santarelli, R.L.; Taché, S.; Naud, N.; Baradat, M.; Jouanin, I.; Surya, R.; Hobbs, D.A.; et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015, 75, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, F.; Freeman, A.; Taché, S.; Van der Meer, R.; Corpet, D.E. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. J. Nutr. 2004, 134, 2711–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, F.; Taché, S.; Petit, C.R.; Van der Meer, R.; Corpet, D.E. Meat and cancer: Haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis 2003, 24, 1683–1690. [Google Scholar] [CrossRef]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose–response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef]
- Fernandez-Cao, J.C.; Arija, V.; Aranda, N.; Bullo, M.; Basora, J.; Martínez-González, M.A.; Díez-Espino, J.; Salas-Salvadó, J. Heme iron intake and risk of new-onset diabetes in a Mediterranean population at high risk of cardiovascular disease: An observational cohort analysis. BMC Public Health 2013, 13, 1042. [Google Scholar] [CrossRef] [Green Version]
- Guéraud, F.; Taché, S.; Steghens, J.-P.; Milkovic, L.; Borovic-Sunjic, S.; Zarkovic, N.; Gaultier, E.; Naud, N.; Héliès-Toussaint, C.; Pierre, F.; et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic. Biol. Med. 2015, 83, 192–200. [Google Scholar] [CrossRef]
- Boléa, G.; Ginies, C.; Vallier, M.-J.; Dufour, C. Lipid protection by polyphenol-rich apple matrices is modulated by pH and pepsin in in vitro gastric digestion. Food Funct. 2019, 10, 3942–3954. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Alary, J.; Debrauwer, L.; Fernandez, Y.; Cravedi, J.-P.; Rao, D.; Bories, G. 1,4-Dihydroxynonene Mercapturic Acid, the Major End Metabolite of Exogenous 4-Hydroxy-2-nonenal, Is a Physiological Component of Rat and Human Urine. Chem. Res. Toxicol. 1998, 11, 130–135. [Google Scholar] [CrossRef]
- Peiro, G.; Alary, J.; Cravedi, J.-P.; Rathahao, E.; Steghens, J.-P.; Guéraud, F. Dihydroxynonene mercapturic acid, a urinary metabolite of 4-hydroxynonenal, as a biomarker of lipid peroxidation. Biofactors 2005, 24, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Peiro, G.; Tache, S.; Cross, A.J.; Bingham, S.A.; Gasc, N.; Gottardi, G.; Corpet, D.E.; Gueraud, F. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2274–2279. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.; Baradat, M.; Jouanin, I.; Debrauwer, L.; Guéraud, F. “Twin peaks”: Searching for 4-hydroxynonenal urinary metabolites after oral administration in rats. Redox Biol. 2015, 4, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.G.; Leonarduzzi, G. 4-hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef]
- Jouanin, I.; Sreevani, V.; Rathahao, E.; Gueraud, F.; Paris, A. Synthesis of the lipid peroxidation product 4-hydroxy-2(E)-nonenal with (13)C stable isotope incorporation. J. Label. Compd. Radiopharm. 2008, 51, 87–92. [Google Scholar] [CrossRef]
- Tanikaga, R.; Nozaki, Y.; Tamura, T.; Kaji, A. Facile Synthesis of 4-Hydroxy-(e)-2-Alkenoic Esters from Aldehydes. Synthesis 1983, 134–135. [Google Scholar] [CrossRef]
- Chevolleau, S.; Noguer-Meireles, M.-H.; Jouanin, I.; Naud, N.; Pierre, F.; Gueraud, F.; Debrauwer, L. Development and validation of an ultra high performance liquid chromatography-electrospray tandem mass spectrometry method using selective derivatisation, for the quantification of two reactive aldehydes produced by lipid peroxidation, HNE (4-hydroxy-2(E)-nonenal) and HHE (4-hydroxy-2(E)-hexenal) in faecal water. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1083, 171–179. [Google Scholar] [CrossRef]
- Anonymous Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 7 October 2020).
- Gueraud, F.; Peiro, G.; Bernard, H.; Alary, J.; Creminon, C.; Debrauwer, L.; Rathahao, E.; Drumare, M.F.; Canlet, C.; Wal, J.M.; et al. Enzyme immunoassay for a urinary metabolite of 4-hydroxynonenal as a marker of lipid peroxidation. Free Radic. Biol. Med. 2006, 40, 54–62. [Google Scholar] [CrossRef]
- Satoh, K.; Yamada, S.; Koike, Y.; Igarashi, Y.; Toyokuni, S.; Kumano, T.; Takahata, T.; Hayakari, M.; Tsuchida, S.; Uchida, K. A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method. Anal. Biochem. 1999, 270, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Waeg, G.; Dimsity, G.; Esterbauer, H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic. Res. 1996, 25, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Borovic, S.; Rabuzin, F.; Waeg, G.; Zarkovic, N. Enzyme-linked immunosorbent assay for 4-hydroxynonenal-histidine conjugates. Free Radic. Res. 2006, 40, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.C.B.; Olier, M.; Ellero-Simatos, S.; Naud, N.; Dupuy, J.; Huc, L.; Taché, S.; Graillot, V.; Levêque, M.; Bézirard, V.; et al. Haem iron reshapes colonic luminal environment: Impact on mucosal homeostasis and microbiome through aldehyde formation. Microbiome 2019, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganjac, M.; Milkovic, L.; Gegotek, A.; Cindric, M.; Zarkovic, K.; Skrzydlewska, E.; Zarkovic, N. The relevance of pathophysiological alterations in redox signaling of 4-hydroxynonenal for pharmacological therapies of major stress-associated diseases. Free Radic. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cagle, B.S.; Crawford, R.A.; Doorn, J.A. Biogenic Aldehyde-Mediated Mechanisms of Toxicity in Neurodegenerative Disease. Curr. Opin. Toxicol. 2019, 13, 16–21. [Google Scholar] [CrossRef]
- Nishikawa, A.; Sodum, R.; Chung, F.L. Acute toxicity of trans-4-hydroxy-2-nonenal in Fisher 344 rats. Lipids 1992, 27, 54–58. [Google Scholar] [CrossRef]
- Chung, F.L.; Tanaka, T.; Hecht, S.S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986, 46, 1285–1289. [Google Scholar]
- Kang, S.C.; Kim, H.-W.; Kim, K.B.; Kwack, S.J.; Ahn, I.Y.; Bae, J.Y.; Lim, S.K.; Lee, B.M. Hepatotoxicity and nephrotoxicity produced by 4-hydroxy-2-nonenal (4-HNE) following 4-week oral administration to Sprague-Dawley rats. J. Toxicol. Environ. Health Part A 2011, 74, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Oarada, M.; Ito, E.; Terao, K.; Miyazawa, T.; Fujimoto, K.; Kaneda, T. The effect of dietary lipid hydroperoxide on lymphoid tissues in mice. Biochim. Biophys. Acta 1988, 960, 229–235. [Google Scholar] [CrossRef]
- Oarada, M.; Miyazawa, T.; Kaneda, T. Distribution of 14C after oral administration of [U-14C]labeled methyl linoleate hydroperoxides and their secondary oxidation products in rats. Lipids 1986, 21, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ashida, H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim. Biophys. Acta 1998, 1393, 349–361. [Google Scholar] [CrossRef]
- Kanazawa, K. Hepatotoxicity Caused by Dietary Secondary Products Originating from Lipid Peroxidation. In Nutritional and Toxicological Consequences of Food Processing; Friedman, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1991; pp. 237–253. ISBN 978-1-4899-2626-5. [Google Scholar]


| % of [13C-HNE]-Protein Adducts | n | % of HNE-Protein Adducts from Orally Given HNE | |
|---|---|---|---|
| Muscle | 7.9 | 1 | 15.8 |
| Liver | 9.3 | 3 | 18.6 |
| Heart | 12.3 | 3 | 24.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, J.; Chevolleau, S.; Noguer-Meireles, M.-H.; Pujos-Guillot, E.; Delosière, M.; Chantelauze, C.; Joly, C.; Blas-y-Estrada, F.; Jouanin, I.; Durand, D.; et al. Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation. Antioxidants 2020, 9, 1293. https://doi.org/10.3390/antiox9121293
Keller J, Chevolleau S, Noguer-Meireles M-H, Pujos-Guillot E, Delosière M, Chantelauze C, Joly C, Blas-y-Estrada F, Jouanin I, Durand D, et al. Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation. Antioxidants. 2020; 9(12):1293. https://doi.org/10.3390/antiox9121293
Chicago/Turabian StyleKeller, Julia, Sylvie Chevolleau, Maria-Helena Noguer-Meireles, Estelle Pujos-Guillot, Mylène Delosière, Céline Chantelauze, Charlotte Joly, Florence Blas-y-Estrada, Isabelle Jouanin, Denys Durand, and et al. 2020. "Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation" Antioxidants 9, no. 12: 1293. https://doi.org/10.3390/antiox9121293
APA StyleKeller, J., Chevolleau, S., Noguer-Meireles, M. -H., Pujos-Guillot, E., Delosière, M., Chantelauze, C., Joly, C., Blas-y-Estrada, F., Jouanin, I., Durand, D., Pierre, F., Debrauwer, L., Theodorou, V., & Guéraud, F. (2020). Heme-Iron-Induced Production of 4-Hydroxynonenal in Intestinal Lumen May Have Extra-Intestinal Consequences through Protein-Adduct Formation. Antioxidants, 9(12), 1293. https://doi.org/10.3390/antiox9121293