Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods
Abstract
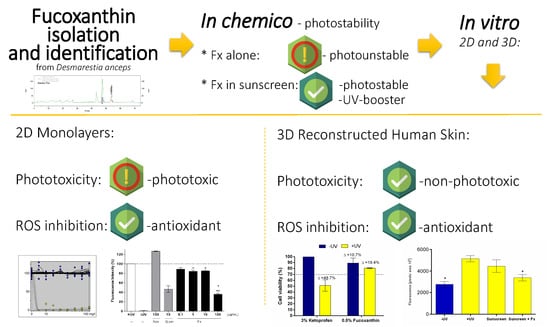
:1. Introduction
2. Materials and Methods
2.1. Alga Material
2.2. Extraction and Fractionation
2.3. Carotenoid Isolation
2.4. Stability
2.4.1. UV Absorption
2.4.2. Photostability Studies
2.5. Toxicity and Efficacy
2.5.1. Phototoxicity Test in 3T3 Mouse Fibroblast (3T3 NRU PT)
2.5.2. Reconstructed Human Skin Model (RHS)
2.5.3. Phototoxicity Test in RHS
2.5.4. Viability Assay
2.5.5. HaCat Antioxidant Activity by Detection of Intracellular ROS Using DCFH2-DA
2.5.6. RHS Antioxidant Activity by Detection of Intracellular ROS Using DCFH2-DA
3. Results
3.1. Extraction and Fractionation
3.2. UV Spectra
3.3. Identification and Isolation of Fucoxanthin
3.4. Photostability Studies
3.5. Phototoxicity in the Monolayer and RHS Assays
3.6. HaCat Antioxidant Activity by Detection of Intracellular ROS using DCFH2-DA
3.7. RHS Antioxidant Activity by Detection of Intracellular ROS Using DCFH2-DA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fourtanier, A.; Moyal, D.; Seite, S. UVA filters in sun-protection products: Regulatory and biological aspects. Photochem. Photobiol. Sci. 2012, 11, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Gaspar, L.R. In vitro photosafety and efficacy screening of apigenin, chrysin and beta-carotene for UVA and VIS protection. Eur. J. Pharm. Sci. 2016, 89, 146–153. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, E.; Baschong, W.; Greci, L. UV-Filter combinations under UV-A exposure: Concomitant quantification of over-all spectral stability and molecular integrity. J. Photochem. Photobiol. B 2007, 87, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Tharmann, J.; Maia Campos, P.M.; Liebsch, M. Skin phototoxicity of cosmetic formulations containing photounstable and photostable UV-filters and vitamin A palmitate. Toxicol. Vitr. 2013, 27, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Adachi, S.; Suzuki, Y. Estrogenic activity of ultraviolet absorbers and the related compounds. Yakugaku Zasshi 2005, 125, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius. Sci. Total. Environ. 2013, 456–457, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [Green Version]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Pandika, M. Looking to Nature for New Sunscreens. ACS Cent. Sci. 2018, 4, 788–790. [Google Scholar] [CrossRef] [Green Version]
- Sohn, M. UV Booster and Photoprotection. In Principles and Practice of Photoprotection; Wang, S.Q., Lim, H.W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 227–245. [Google Scholar]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccioni, G.; D’Orazio, N.; Franceschelli, S.; Speranza, L. Marine carotenoids and cardiovascular risk markers. Mar. Drugs 2011, 9, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of Fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef]
- Ceridono, M.; Tellner, P.; Bauer, D.; Barroso, J.; Alépée, N.; Corvi, R.; De Smedt, A.; Fellows, M.D.; Gibbs, N.K.; Heisler, E.; et al. The 3T3 neutral red uptake phototoxicity test: Practical experience and implications for phototoxicity testing--the report of an ECVAM-EFPIA workshop. Regul. Toxicol. Pharmacol. 2012, 63, 480–488. [Google Scholar] [CrossRef]
- ICH. Photosafety Evaluation of Pharmaceuticals. In S10 Guideline; ICH: Geneva, Switzerland, 2013. [Google Scholar]
- Catarino, C.M.; do Nascimento Pedrosa, T.; Pennacchi, P.C.; de Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; de Moraes Barros, S.B.; Maria-Engler, S.S. Skin corrosion test: A comparison between reconstructed human epidermis and full thickness skin models. Eur. J. Pharm. Biopharm. 2018, 125, 51–57. [Google Scholar] [CrossRef]
- Cambon, M.; Issachar, N.; Castelli, D.; Robert, C. An in vivo method to assess the photostability of UV filters in a sunscreen. J. Cosmet. Sci. 2001, 52, 1–11. [Google Scholar]
- Whitehead, K.; Hedges, J.I. Photodegradation and photosensitization of mycosporine-like amino acids. J. Photochem. Photobiol. B 2005, 80, 115–121. [Google Scholar] [CrossRef]
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability evaluation of five UV-filters, trans-resveratrol and beta-carotene in sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89. [Google Scholar] [CrossRef]
- Maia-Campos, P.M.B.G.; Benevenuto, C.G.; Calixto, L.S.; Melo, M.O.; Pereira, K.C.; Gaspar, L.R. Palmariapalmata, Cichorium intybus, and Medicago sativa extracts in cosmetic formulations: An integrated approach of in vitro toxicity and in vivo acceptability studies. Cutan. Ocul. Toxicol. 2019, 38, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Optimizing the spectral absorption profile of sunscreens. Int. J. Cosmet. Sci. 2017, 39, 90–92. [Google Scholar] [CrossRef]
- Liebsch, M.; Spielmann, H.; Pape, W.; Krul, C.; Deguercy, A.; Eskes, C. UV-induced effects. Altern. Lab. Anim. 2005, 33 (Suppl. S1), 131–146. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 432: In vitro 3T3 NRU Phototoxicity Test, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faião-Flores, F.; de Araújo Crepaldi, M.C.; Dos Santos, M.F.; de Moraes Barros, S.B.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Kandarova, H.; Liebsch, M. The EpiDermTM Phototoxicity Test (EpiDermTM H3D-PT) In Alternatives for Dermal Toxicity Testing; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Liebsch, M.; Traue, D.; Barrabas, C.; Spielmann, H.; Gerberick, G.F.; Cruse, L.; Diembeck, W.; Pfannenbecker, U.; Spieker, J.; Holzhutter, H.-G.; et al. Prevalidation of the epiderm™ phototoxicity test. In Proceedings of International Scientific Conference, Alternatives to Animal Testing, 2nd ed.; CPL Press: Brussels, Belgium, 1999; pp. 160–166. [Google Scholar]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Pygmalion, M.J.; Ruiz, L.; Popovic, E.; Gizard, J.; Portes, P.; Marat, X.; Lucet-Levannier, K.; Muller, B.; Galey, J.B. Skin cell protection against UVA by Sideroxyl, a new antioxidant complementary to sunscreens. Free Radic. Biol. Med. 2010, 49, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.A.D.; Oliveira de Souza, R.; Ghislain Rogez, H.L.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit anti aging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C.; Golebiewski, C.; Bernerd, F. Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS ONE 2014, 9, e105263. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.; Gratz, K.; Liebel, F.; Southall, M.; Garay, M.; Bhattacharyya, S.; Simon, N.; Vander Zanden, M.; Van Winkle, K.; Pirnstill, J.; et al. The StrataTest® human skin model, a consistent in vitro alternative for toxicological testing. Toxicol. Vitr. 2010, 24, 2021–2029. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Hojerová, J.; Medovcíková, A.; Mikula, M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int. J. Pharm. 2011, 408, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Sik, R.H. A photochemical study of cells loaded with 2′,7′-dichlorofluorescin: Implications for the detection of reactive oxygen species generated during UVA irradiation. Free Radic. Biol. Med. 2003, 34, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef] [PubMed]
- Rautenberger, R.W.C.; Bischof, K. Acclimation to UV radiation and antioxidative defence in the endemic Antarctic brown macroalga Desmarestia anceps along a depth gradient. Polar Biol. 2013, 36, 1779–1789. [Google Scholar] [CrossRef]
- Santos, A.J.; Miranda, M.S.; Esteves da Silva, J.C. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res. 2012, 46, 3167–3176. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- ICH. Photostability Testing of New Drug Substances and Products. In Q1B Guideline; ICH: Geneva, Switzerland, 1996. [Google Scholar]
- Gaspar, L.R.; Kawakami, C.M.; Benevenuto, C.G. Overview on the Current Status of Available Test Methods and Additional Promising Methods for Assessing UV-Induced Effects. In Alternatives for Dermal Toxicity Testing; ©Springer International Publishing AG: Cham, Switzerland, 2017; p. 463. [Google Scholar]
- Kejlová, K.; Jírová, D.; Bendová, H.; Kandárová, H.; Weidenhoffer, Z.; Kolárová, H.; Liebsch, M. Phototoxicity of bergamot oil assessed by in vitro techniques in combination with human patch tests. Toxicol. Vitr. 2007, 21, 1298–1303. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Ruan, J.; Yang, Z.; Wang, C.; Hong, Z.; Zuo, Z. Protective effects of fucoxanthin and fucoxanthinol against tributyltin-induced oxidative stress in HepG2 cells. Environ. Sci. Pollut. Res. Int. 2018, 25, 5582–5589. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [Green Version]
- Tavares, R.S.N.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.K.; Obermueller-Jevic, U.C. UV light, beta-carotene and human skin—Beneficial and potentially harmful effects. Arch. Biochem. Biophys. 2001, 389, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, C. Beta-carotene in dermatology: Does it help? Acta Dermatovenerol. APA 2008, 17, 160–162. [Google Scholar]
- Zhang, P.; Omaye, S.T. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol. Vitr. 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Fernández, E.; Fajarí, L.; Rodríguez, G.; Cócera, M.; Moner, V.; Barbosa-Barros, L.; Kamma-Lorger, C.S.; de la Maza, A.; López, O. Reducing the Harmful Effects of Infrared Radiation on the Skin Using Bicosomes Incorporating β-Carotene. Skin Pharmacol. Physiol. 2016, 29, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]






| Position | 1H (δ; mult; J-Hz) | 13C (δ) | |||
|---|---|---|---|---|---|
| Literature * | Fucoxanthin | Literature * | Fucoxanthin | ||
| 1 | 35.6 | 35.5 | |||
| 2 | ax eq | 1.36 dd (8.7; 14.2) | 1.36 m | 46.9 | 46.9 |
| 3 | 3.80 m | 3.84 m | 64.2 | 64.0 | |
| 4 | ax eq | 1.77 dd (8.7; 14.2) 2.29 dd (2.9; 17.8) | 1.77 dd (9.1; 13.9) 2.30 t (13.5) | 41.5 | 41.3 |
| 5 | 66.0 | 67.0 | |||
| 6 | 66.9 | 66.8 | |||
| 7 | 3.64 d (20.4) 2.59 d (20.4) | 3.65 d (18.4) 2.60 d (18.4) | 40.6 | 40.0 | |
| 8 | 197.7 | 197.7 | |||
| 9 | 134.3 | 134.4 | |||
| 10 | 7.14 d (12.8) | 7.15 d (10.4) | 139.0 | 139.0 | |
| 11 | 6.58 m | 6.57 m | 123.2 | ||
| 12 | 6.66 t (12.8) | 6.66 t (11.3) | 144.9 | 144.9 | |
| 13 | 135.3 | ||||
| 14 | 6.40 d (11.6) | 6.41 d (11.7) | 136.6 | 136.4 | |
| 15 | 6.67 m | 6.66 m | 129.3 | ||
| 16 | −Me | 1.02 s | 1.03 s | 24.9 | 24.4 |
| 17 | −Me | 0.95 s | 0.96 s | 28.0 | 28.0 |
| 18 | 1.21 s | 1.21 s | 21.0 | 21.0 | |
| 19 | 1.93 s | 1.93 s | 11.7 | 11.7 | |
| 20 | 1.98 s | 1.99 s | 12.8 | 12.7 | |
| C-3’OAc | −Me | 2.03 s | 2.03 s | 21.3 | 21.6 |
| 1’ | 35.0 | 35.5 | |||
| 2’ | ax eq | 1.41 dd (10.4; 14.9) 2.00 dd (2.9; 14.9) | 1.42 d (11.9) 1.99 m | 45.2 | 45.3 |
| 3’ | 5.37 tt (8.8; 12.0) | 5.38 m | 67.8 | 67.7 | |
| 4’ | ax eq | 1.53 dd (10.4; 14.9) 2.29 dd (2.9; 17.8) | 1,51 t (11.9) 2.30 m | 45.1 | 45.1 |
| 5’ | 72.6 | 72.5 | |||
| 6’ | 117.3 | 117.3 | |||
| 7’ | 202.2 | ||||
| 8’ | 6.04 s | 6.05 s | 103.2 | 103.1 | |
| 9’ | 132.4 | 132.3 | |||
| 10’ | 6.12 d (11.6) | 6.13 d (11.1) | 128.4 | 128.1 | |
| 11’ | 6.71 t (12.0) | 6.75 t (12.1) | 125.5 | ||
| 12’ | 6.34 d (11.6) | 6.35 d (15.0) | 137.0 | 136.4 | |
| 13’ | 138.0 | ||||
| 14’ | 6.26 d (11.6) | 6.27 d (11.5) | 132.0 | ||
| 15’ | 6.71 dd (12.0; 14.2) | 6.75 t (13.7) | 132.4 | ||
| 16’ | −Me | 1.37 | 1.38 s | 29.0 | 29.2 |
| 17’ | −Me | 1.065 | 1.07 s | 31.9 | 32.0 |
| 18’ | 1.345 | 1.34 s | 31.1 | 31.2 | |
| 19’ | 1.805 | 1.81 s | 13.9 | 13.9 | |
| 20’ | 1.985 | 1.98 s | 12.8 | 12.7 | |
| 21 | 170.0 | 170.4 | |||
| Sample | Irradiation Dose (J/cm2) | Mean of the Reduction of Absorbance after Irradiation (%) | ||
|---|---|---|---|---|
| UVB | UVA | VIS | ||
| Crude Extract | 27.5 | 28.5 | 43.2 | 33.7 |
| Fraction F15 * | 27.5 | 4.0 | 44.0 | 49.0 |
| Fucoxanthin Isolated | 6 ** | 0.0 | 35.0 | 21.0 |
| Fucoxanthin in Sunscreen | 27.5 | 5.8 | 16.5 | NE |
| Chemical | IC50 − UV | IC50 + UV | MPE | PIF | Result |
|---|---|---|---|---|---|
| Extract | 2.76 | 3.05 | −0.014 | 0.90 | cytotoxic |
| Fraction F15a | 26.12 | 4.45 | 0.343 | 7.08 | photo/cytotoxic |
| 25.22 | 2.59 | 0.478 | 16.13 | photo/cytotoxic | |
| Fucoxanthin | - | 2.77 | 0.920 | 48.21 | phototoxic |
| - | 5.91 | 0.915 | 17.04 | phototoxic | |
| Positive Control (Norfloxacin) | - | 2.487 | 0.615 | 43.75 | phototoxic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, R.S.N.; Kawakami, C.M.; Pereira, K.d.C.; do Amaral, G.T.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods. Antioxidants 2020, 9, 328. https://doi.org/10.3390/antiox9040328
Tavares RSN, Kawakami CM, Pereira KdC, do Amaral GT, Benevenuto CG, Maria-Engler SS, Colepicolo P, Debonsi HM, Gaspar LR. Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods. Antioxidants. 2020; 9(4):328. https://doi.org/10.3390/antiox9040328
Chicago/Turabian StyleTavares, Renata Spagolla Napoleão, Camila Martins Kawakami, Karina de Castro Pereira, Gabriela Timotheo do Amaral, Carolina Gomes Benevenuto, Silvya Stuchi Maria-Engler, Pio Colepicolo, Hosana Maria Debonsi, and Lorena Rigo Gaspar. 2020. "Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods" Antioxidants 9, no. 4: 328. https://doi.org/10.3390/antiox9040328
APA StyleTavares, R. S. N., Kawakami, C. M., Pereira, K. d. C., do Amaral, G. T., Benevenuto, C. G., Maria-Engler, S. S., Colepicolo, P., Debonsi, H. M., & Gaspar, L. R. (2020). Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods. Antioxidants, 9(4), 328. https://doi.org/10.3390/antiox9040328