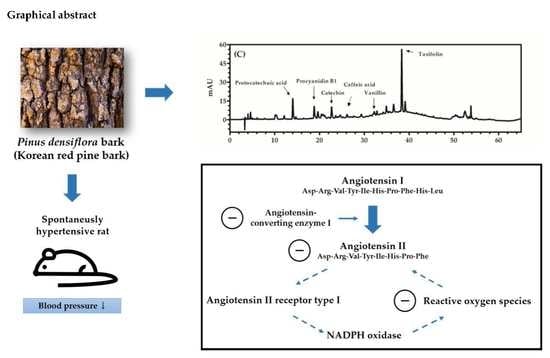
Antihypertensive Effects of Polyphenolic Extract from Korean Red Pine (Pinus densiflora Sieb. et Zucc.) Bark in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Quantification of Phenolics Using HPLC
2.3. Animals
2.4. Oral Administration of KRPBE
2.5. Measurement of Blood Pressure
2.6. Collection of Tissue and Serum
2.7. Measurement of ACE Activity
2.8. Measurement of Angiotensin II Content
2.9. Measurement of MDA Content
2.10. Statistical Analysis
3. Results
3.1. Quantification of Phenolics Using HPLC
3.2. Effect of KRPBE on Blood Pressure in WKRs and SHRs
3.3. ACE Activity in the Lungs, Kidneys, and Serum of WKRs and SHRs
3.4. Angiotensin II Content in Lungs, Kidneys, and Serum of WKRs and SHRs
3.5. MDA Content in Lungs, Kidneys, and Serum of WKRs and SHRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| ACEI | angiotensin-converting enzyme inhibitor |
| AT1 | angiotensin II type 1 |
| CAP | captopril |
| DBP | diastolic blood pressure |
| HHL | N-hippuryl-His-Leu hydrate |
| HL | His-Leu |
| HPLC | high-performance liquid chromatography |
| IL | interleukin |
| KRPBE | Korean red pine bark extract |
| MDA | malondialdehyde |
| RAS | renin-angiotensin system |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| SHR | spontaneously hypertensive rat |
| WKR | Wistar-Kyoto rat |
References
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. A Global Brief on Hypertension-Silent Killer, Global Public Health Crisis. Available online: https://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed on 25 March 2020).
- Peach, M.J. Renin-Angiotensin system: Biochemistry and mechanisms of action. Physiol. Rev. 1977, 57, 313–370. [Google Scholar] [CrossRef]
- Drayer, J.I.; Weber, M.A. Monotherapy of essential hypertension with a converting-enzyme inhibitor. Hypertension 1983, 5, III108–III113. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, H.; Henning, F.; Tager, T.; Schellberg, D.; Grundtvig, M.; Goode, K.; Corletto, A.; Kazmi, S.; Hole, T.; Katus, H.A.; et al. Comparative effectiveness of enalapril, lisinopril, and ramipril in the treatment of patients with chronic heart failure: A propensity score-Matched cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Gavras, H.; Gavras, I. Angiotensin converting enzyme inhibitors. Properties and side effects. Hypertension 1988, 11, II37–II41. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, K.; Aihara, M.; Osawa, J.; Naito, S.; Ikezawa, Z. Sulfhydryl drug-induced eruption: A clinical and histological study. J. Dermatol. 1990, 17, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Filho, J.M.; Martins, V.K.M.; Rabelo, L.A.; Moura, M.D.; Silva, M.S.; Cunha, E.V.L.; Souza, M.F.V.; Almeida, R.N.; Medeiros, I.A. Natural products inhibitors of the angiotensin converting enzyme. Rev. Bras. Farmacogn. 2006, 16, 421–446. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhou, Q.G.; Nie, J.; Wang, G.B.; Liu, Y.; Zhou, Z.M.; Hou, F.F. Albumin overload activates intrarenal renin-Angiotensin system through protein kinase C and NADPH oxidase-Dependent pathway. J. Hypertens. 2011, 29, 1411–1421. [Google Scholar] [CrossRef]
- Morato, M.; Reina-Couto, M.; Pinho, D.; Albino-Teixeira, A.; Sousa, T. Regulation of the renin-angiotensin-Aldosterone system by reactive oxygen species. In Renin-Angiotensin System-Past, Present and Future; Tolekova, A.N., Ed.; IntechOpen: London, UK, 2017; Volume 2020, pp. 119–157. [Google Scholar]
- Kim, J.H.; Kim, H.; Kim, Y.H.; Chung, W.S.; Suh, J.K.; Kim, S.J. Antioxidant effect of captopril and enalapril on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Mun, S.P. Liquefaction of pine bark using phenol and lower alcohols with methanesulfonic acid catalyst. J. Ind. Eng. Chem. 2002, 8, 359–364. [Google Scholar]
- Korea Forest Service. Statistical Yearbook of Forestry 2018. Available online: https://www.forest.go.kr/newkfsweb/cop/bbs/selectBoardArticle.do?nttId=3122752&bbsId=BBSMSTR_1064&pageIndex=1&pageUnit=10&searchtitle=title&searchcont=&searchkey=&searchwriter=&searchdept=&searchWrd=&ctgryLrcls=&ctgryMdcls=&ctgrySmcls=&ntcStartDt=&ntcEndDt=&orgId=kfs&mn=KFS_02_03_06 (accessed on 25 March 2020).
- Mun, S.P.; Ku, C.S.; Kim, J.P. Adsorption of metal and uranyl ions onto amidoximated Pinus densiflora bark. Wood Sci. Technol. 2010, 44, 283–299. [Google Scholar] [CrossRef]
- Kim, J.W.; Im, S.; Jeong, H.R.; Jung, Y.S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.-O. Neuroprotective effects of Korean red pine (Pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.B.; Zhang, Y.T.; Du, Y.; Jiang, Y.W. Preventive effects of catechins on cardiovascular disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arutyunyan, T.V.; Korystova, A.F.; Kublik, L.N.; Levitman, M.; Shaposhnikova, V.V.; Korystov, Y.N. Effects of taxifolin on the activity of angiotensin-Converting enzyme and reactive oxygen and nitrogen species in the aorta of aging rats and rats treated with the nitric oxide synthase inhibitor and dexamethasone. Age 2013, 35, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Ikari, N.; Ohta, H.; Niwa, M.; Nonaka, G.-I.; Nishioka, I.; Ozaki, M. Inhibitory effects of condensed tannins on angiotensin converting enzyme. Jpn. J. Pharmacol. 1987, 43, 242–246. [Google Scholar] [CrossRef]
- Wagner, H.; Elbl, G. ACE-Inhibitory procyanidins from Lespedeza capitata. Planta Med. 1992, 58, 297. [Google Scholar] [CrossRef] [Green Version]
- Schwager, S.L.; Carmona, A.K.; Sturrock, E.D. A high-Throughput fluorimetric assay for angiotensin I-Converting enzyme. Nat. Protoc. 2006, 1, 1961–1964. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, L.; Roy, S.K.; Cornish, K.G.; Zucker, I.H. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ. Res. 2008, 103, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Nakamura, K.; Matsukura, T.; Nakamura, K. Vascular angiotensin converting enzyme activity in spontaneously hypertensive rats and its inhibition with cilazapril. J. Hypertens. 1988, 6, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Lahiri, S.; Mohan, D.; Valicherla, G.R.; Gupta, A.P.; Riyazuddin, M.; Kumar, S.; Maurya, R.; Hanif, K.; Gayen, J.R. Evaluation of anti-Hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt- and L-NAME-Induced hypertensive rats. J. Ethnopharmacol. 2016, 193, 555–565. [Google Scholar] [CrossRef]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef]
- Hackenthal, E.; Paul, M.; Ganten, D.; Taugner, R. Morphology, physiology, and molecular biology of renin secretion. Physiol. Rev. 1990, 70, 1067–1116. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim. Biophys. Acta 1971, 250, 261–265. [Google Scholar] [CrossRef]
- Laragh, J.J. Two forms of vasoconstriction in systemic hypertension. Am. J. Cardiol. 1987, 60, G82–G93. [Google Scholar] [CrossRef]
- Farmer, E.E.; Davoine, C. Reactive electrophile species. Curr. Opin. Plant. Biol. 2007, 10, 380–386. [Google Scholar] [CrossRef]
- Anupama, V.; George, M.; Dhanesh, S.B.; Chandran, A.; James, J.; Shivakumar, K. Molecular mechanisms in H2O2-Induced increase in AT1 receptor gene expression in cardiac fibroblasts: A role for endogenously generated angiotensin II. J. Mol. Cell. Cardiol. 2016, 97, 295–305. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Yin, J.; Tam, L.T.; Youn, S.H.; Ahn, H.S.; Kwon, S.H.; Min, B.K.; Yun, S.H.; An, Y.E.; Lee, M.W. Quantitative analysis of taxifolin, (+)-catechin and procyanidin B1 from the preparation of Pinus densiflora (PineXol®). Korean J. Physiol. Pharmacol. 2016, 47, 246–250. [Google Scholar]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Paixao, J.; Dinis, T.C.; Almeida, L.M. Malvidin-3-glucoside protects endothelial cells up-Regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem. Biol. Interact. 2012, 199, 192–200. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Cui, Y.; Zhou, H.; Xu, D.; Shan, T.; Zhang, F.; Guo, Y.; Chen, Y.; Wu, D. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol. Appl. Pharmacol. 2015, 287, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Dashore, A.; Choudhary, S.D. Captopril induced pemphigus vulgaris. Indian J. Dermatol. Venereol. Leprol. 1987, 53, 293–294. [Google Scholar]
- Karna, E.; Szoka, L.; Palka, J.A. Captopril-Dependent inhibition of collagen biosynthesis in cultured fibroblasts. Pharmazie 2010, 65, 614–617. [Google Scholar]
- Stuermer, K.E.; Besser, M.; Terberger, N.; Bachmann, S.H.; Severing, A.-L. Side effects of frequently used antihypertensive drugs on wound healing in vitro. Skin Pharmacol. Phys. 2018, 32, 162–172. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, K.E.; Nahar, L.; Sarker, D.S. Wound healing and antioxidant properties: Do they coexist in plants? Free Rad. Antiox. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Blazsó, G.; Gábor, M.; Schönlau, F.; Rohedewald, P. Pycnogenol® accelerates wound healing and reduces scar formation. Phytother. Res. 2004, 18, 579–581. [Google Scholar] [CrossRef]
- Cetin, E.O.; Yesil-Celiktas, O.; Cavusoglu, T.; Demirel-Sezer, E.; Akdemir, O.; Uyanikgil, Y. Incision wound healing activity of pine bark extract containing topical formulations: A study with histopathological and biochemical analyses in albino rats. Pharmazie 2013, 68, 75–80. [Google Scholar]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Subramanian, V.; Rao, J.R.; Nair, B.U.; Ramasami, T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol. 2005, 37, 47–53. [Google Scholar] [CrossRef] [PubMed]




| Phenolics | Concentration (mg/g Dry Weight) |
|---|---|
| Protocatechuic acid | 3.99 ± 0.21 |
| Procyanidin B1 | 23.78 ± 1.17 |
| Catechin | 9.06 ± 0.42 |
| Caffeic acid | 0.29 ± 0.01 |
| Vanillin | 0.41 ± 0.01 |
| Taxifolin | 6.38 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.J.; Hwang, E.-S.; Kim, M.-J.; Park, J.-H.; Kim, D.-O. Antihypertensive Effects of Polyphenolic Extract from Korean Red Pine (Pinus densiflora Sieb. et Zucc.) Bark in Spontaneously Hypertensive Rats. Antioxidants 2020, 9, 333. https://doi.org/10.3390/antiox9040333
Kim KJ, Hwang E-S, Kim M-J, Park J-H, Kim D-O. Antihypertensive Effects of Polyphenolic Extract from Korean Red Pine (Pinus densiflora Sieb. et Zucc.) Bark in Spontaneously Hypertensive Rats. Antioxidants. 2020; 9(4):333. https://doi.org/10.3390/antiox9040333
Chicago/Turabian StyleKim, Kwan Joong, Eun-Sang Hwang, Min-Jeong Kim, Ji-Ho Park, and Dae-Ok Kim. 2020. "Antihypertensive Effects of Polyphenolic Extract from Korean Red Pine (Pinus densiflora Sieb. et Zucc.) Bark in Spontaneously Hypertensive Rats" Antioxidants 9, no. 4: 333. https://doi.org/10.3390/antiox9040333
APA StyleKim, K. J., Hwang, E. -S., Kim, M. -J., Park, J. -H., & Kim, D. -O. (2020). Antihypertensive Effects of Polyphenolic Extract from Korean Red Pine (Pinus densiflora Sieb. et Zucc.) Bark in Spontaneously Hypertensive Rats. Antioxidants, 9(4), 333. https://doi.org/10.3390/antiox9040333